Chapter 10
Using oral medications, infusions, and injections for differential diagnosis of orofacial pain
10.1 Diagnostic Dilemmas in Orofacial Pain
There are many different reasons for pain in the orofacial region; some of these problems are very difficult to differentiate and pose a challenge for the clinician. For example, when a patient has jaw pain and limited opening, it could be due to an intracapsular disorder (disk derangement) or an extracapsular disorder (trismus). Determining the exact cause of a restricted jaw opening is not always easy and trying to identify the causation with advanced imaging such as computerized tomography (CT) and magnetic resonance imaging (MRI) can be expensive for the patient and may not be covered by health insurance policies. An alternate and faster diagnostic procedure is to inject an anesthetic solution into the painful joint to see if the patient can then open his or her mouth. Using injectable or even oral medications to assist in the diagnosis of jaw locking is one example, but there are other examples of diagnostic dilemmas that can be evaluated with medications. This chapter focuses on how various medications can be used as tests to diagnose orofacial pain problems.
10.1.A Source of Pain Is Not Always Visible on Imaging Studies
According to the National Institute of Neurologic Disorders and Stroke (NINDS) website, there is no test or device available that can measure the amount of pain or image the area of pain or locate the pain precisely. Currently, the best aid to diagnosis in chronic pain is the patient’s own description of the type, duration, or location of pain.1 These sentences capture one of the major frustrations that is inherent in being a diagnostician specializing in pain disorders, namely, that pain without an obvious organic pathology is not visible on a radiograph or standard MRI image. Of course functional MRI (fMRI) images can show you what areas of the brain are activated by experimental pain, but these images are not specific to the diagnosis and will not work in chronic pain since there is no prepain baseline to compare against. Because most pain disorders are without an incontrovertible physical examination finding or image-based “gold standard,” gathering a careful history is critical to the process. However, once a diagnosis has been formulated, it is logical to have a test of the correctness of this theory. Most of the time, proof-of-concept testing is done with treatment and, for neuropathic pain in particular, with medications.
10.1.B Accuracy of Diagnostic Tests
An experienced diagnostician knows that diagnostic tests are rarely infallible and this is just as true in the field of orofacial pain as it is for any other area of medicine. The diagnostician, like a good investigative journalist, should insist upon having at least two sources (positive tests) to arrive at a diagnosis. For instance, a positive radiograph showing arthritic change in the temporomandibular joint (TMJ), palpation findings demonstrating capsular pain, plus reduction in pain with an anti-inflammatory drug all lead to the conclusion that arthritis associated inflammation is the pain source. The same process needs to be put forth with medications as diagnostic tests, in that they should be only one piece of information and must be used in combination with available examination, history, and imaging data. For example, it is common to give antibiotics to patients with a toothache (even when the tooth has no obvious signs of infection clinically or radiographically). One conclusion that could be made if the pain decreased as a result of the antibiotic is that the patient has an infection. This conclusion is not always true, however, since it is known that some antibiotics may be analgesics.2,3 See Chapter 9 for more information on antibiotics as analgesics.
10.1.C Effect of Inactive Substances in Differential Diagnosis of Orofacial Pain
Certainly, all clinicians will tell you that certain patients are more responsive to medications than others. In fact, some patients are labeled as placebo-responders,4 which implies that they respond positively with clinical improvement to an inactive agent. Negative placebo responders are called nocebo-responders,5 which means they experience adverse effects following the administration of an inactive substance. Such responders pose a problem in using medications for diagnosing pain disorders.
10.1.D Withdrawal of Medications As a Diagnostic Test
There are many situations where taking a patient off a medication might be a valuable diagnostic test. Let us assume a patient presents with a chronic daily headache and is using analgesics multiple times a day to try to suppress the pain. One possibility in their diagnosis is that they have a medication overuse headache (MOH).6 Withdrawal of the analgesics to improve the pain seems paradoxical, but if the MOH diagnosis is correct, this is proof of the diagnosis.7 Another scenario where medication withdrawal will confirm the diagnosis is face-and-jaw pain in a patient, caused by a selective serotonin reuptake inhibitor (SSRI) that is causing a dystonic extrapyramidal reaction affecting the jaw muscles.8
10.2 Local Anesthetic Use in Orofacial Pain
Local anesthetics act to selectively block sodium channels in the nerve fibers and increase the threshold for spontaneous firing of the nerves. Occasionally, nerve blocks are used diagnostically for facial pain. An example is the use of a nerve block to assess chronic orodental pain of possible neuropathic origin. In this situation, if the pain does not diminish as expected after local infiltration of 2% lidocaine in the area, the neuropathic changes are considered to be more central (affecting the second- and third-order neurons). The conclusions made as a result of a failed dental anesthesia is that patients will require systemic (usually anticonvulsant) medications in addition to the topical anesthetics to manage the chronic pain. Whether they are used diagnostically or therapeutically, nerve blocks have an associated risk in that sometimes the nerve can be aggravated by the injection. This was described in a case series of 83 patients (55 women and 28 men) who were referred to a tertiary care center with permanent alterations of the trigeminal nerve (sometimes painful and sometimes paraesthesia) after an inferior alveolar nerve block.9 Most of these cases involved the lingual nerve (79%) and fewer in the inferior alveolar nerve (21%). They concluded that, while rare, occasionally an inferior alveolar nerve block can result in increased activity of the nerve. It is possible that some of these patients developed neuropathic changes secondary to direct nerve injury during the inferior alveolar block.
10.2.A Auriculotemporal Nerve Block (Temporomandibular Joint Injection)
The auriculotemporal (AT) nerve is a branch of the mandibular division of the trigeminal nerve that supplies the TMJ and preauricular skin. Sensitization of the AT nerve may result in chronic dull aching or burning pain that is unresolved with routine anti-inflammatory medications. This condition is referred to as AT neuropathy and is often difficult to diagnose. AT nerve blocks are diagnostic blocks that are performed to differentiate between inflammation-mediated pain emanating from the TMJ and neuropathic pain originating from the nerve itself.
This nerve block is simple and easy to learn and perform. The injection setup is shown in Figure 10.1 and includes a 3-mL disposable Luer lock syringe, a 23-gauge needle to withdraw the solutions, a 27-gauge needle to inject the joint, 2% lidocaine without epinephrine, triamcinolone acetonide (40 mg/mL), and alcohol or iodine pads. The triamcinolone acetonide (a corticosteroid) is mixed with lidocaine and used for cases where the pain is suspected to have an inflammatory cause, for example, TMJ arthritis (see Sec. 19.3.B). The injection is performed in the superior joint space of the TMJ after wiping the preauricular skin with iodine or alcohol (Fig. 10.2). An equivocal result following a block is indicative of sensitization and neuropathic changes in the AT nerve. The next step will be to control the pain using 5% topical lidocaine patches, topical lidocaine in pluronic lecithin organogel (PLO), or centrally acting medications such as neurontin or pregabalin.
Figure 10.1 Setup for auriculotemporal (AT) nerve block or temporomandibular joint (TMJ) injection.
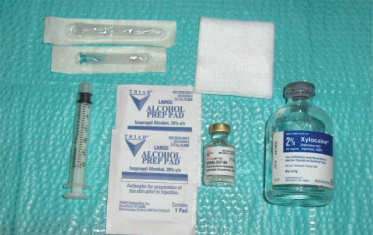
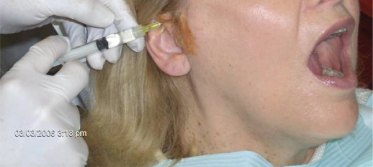
Figure 10.2 Right-sided AT nerve block or TMJ injection using 1.5 cc of 2% lidocaine without epinephrine after preparing preauricular skin with povidone–iodine swab.
A recent study evaluated the efficacy of AT nerve blocks on somatosensory function in the TMJ by injecting bupivacaine in 14 healthy volunteers with no history of TMJ disorders.10 The results of this study showed that AT nerve blocks with local anesthetic caused a significant decrease over time in the pinprick sensitivity—which, however, did not differ significantly from saline (placebo injection in the opposite joint). There was a significant increase in the pressure pain thresholds at 30 minutes and pressure pain tolerance at 30 minutes, 1 hour, and 2 hours after bupivacaine injections compared with saline.
10.2.B Sphenopalatine Ganglion Block
The blockade of the sphenopalatine ganglion has been used to manage intractable headaches such as cluster headaches and facial pain presenting with autonomic signs such as rhinorrhea, lacrimation and, nasal congestion. The block is more often used as a last resort for managing intractable facial pain than for diagnostic purposes. The sphenopalatine ganglion is the largest peripheral parasympathetic ganglion having multiple connections to general sensory fibers, and the internal carotid plexus without synapses. There are generally three approaches to block this ganglion: (1) transnasal application of topical anesthetic with a cotton-tipped applicator to the nasopharyngeal mucosa posterior to the middle turbinate; (2) transoral approach with a curved dental needle up to the sphenopalatine foramen through the posterior palatine canal; and (3) the lateral approach with a straight needle to the pterygopalatine fossa through the infratemporal fossa.11 The transnasal application of topical anesthetic is the simplest and the most tolerable technique among the three approaches. However, the diffusion of topical anesthetic to the ganglion is unpredictable and the blockade is not durable with this approach. A new approach of transnasally injecting the sphenopalatine ganglion was described by Yang and Oraee in 2006.12 The injection was done with triamcinolone 20 mg in 1.5 mL of 0.2% ropivacaine. The technique was reported to be safe and effective for short-term management of intractable cluster headache pain in one patient. Four-percent lidocaine has been used in studies to block the sphenopalatine ganglion for management of myofascial pain of the head and neck or fibromyalgia pain. The blocks were found to be no better than placebo in these cases.13,14 As a general rule, this block must always be performed by an experienced anesthesiologist or pain specialist.
10.2.C Stellate Ganglion Block
The cervical sympathetic chain is composed of superior, middle, intermediate, and inferior cervical ganglia. However, in approximately 80% of the population, the inferior cervical ganglion is fused with the first thoracic ganglion, forming the stellate ganglion also known as the cervicothoracic ganglion.15 Peripheral sympathetic blocks, though popular among pain specialists, are not supported by evidence in the scientific literature. First, the actual success rate of blocking the sympathetic activity with these blocks is not known. Second, no placebo-controlled trials have been published. Third, the mechanism of pain relief when achieved may be local anesthetic activity on peripheral somatic nerve fibers and not sympathetic fibers via local anesthetic systemic concentration or local spillage.16–18 In fact, patients who have reported transient pain relief with sympathetic block may also report similar degrees of pain relief with intravenous lidocaine infusion and then obtain chronic relief with oral mexiletine (see Sec. 10.5.A). Nevertheless, sympathetically mediated pain (SMP/CRPS type I) of the head, neck, and upper arm can be distinguished from other overlapping pain disorders by performing a stellate ganglion block. Successful block of sympathetic fibers to the head is indicated by the appearance of Horner’s syndrome (ptosis, miosis, enophthalmos, anhidrosis of the neck and face) and relief of pain. Therefore, this block must always be performed by an experienced anesthesiologist or pain specialist.19
10.2.D Occipital Nerve Block
The greater and lesser occipital nerves supply most of the posterior scalp and are the source of pain in occipital headaches, occipital neuralgia, and other painful conditions affecting the back of the head.20 Occipital nerve block is a very safe block that can be performed to distinguish between occipital neuralgia, occipital headaches, and musculoskeletal pain.
10.2.E Cervical Plexus Block
Pain originating from the cervical plexus may refer to other sites of the orofacial complex especially to the posterior aspect of the head, and is implicated in the pathogenesis of cervicogenic headaches or C2 neuralgia. A thorough clinical examination of the neck and associated structures along with appropriate imaging to visualize the cervical joints is a must to rule out obvious pathological sources of pain such as lesions and arthritis. The block has been reported to be effective in relieving orofacial pain originating from the cervical region. Significant pain relief was obtained with the cervical plexus block compared with regional anesthesia or trigger-point injections. It has been suggested that the block may be effective in the differential diagnosis of pain originating from deep cervical muscles and nerves.21
10.2.F Local Anesthetic Blocks for Trigeminal Neuralgia Pain
Trigeminal neuralgia pain is often diagnosed clinically by the presence of trigger zones and unique characteristic features of unilateral, episodic, paroxysmal, lancinating pains that typically last from a few seconds to minutes with multiple attacks during the day. In general, the diagnosis can be established with a thorough history. Occasionally, patients may present with pain attacks, preventing the practitioner from obtaining a thorough history or performing an examination. In such cases, diagnostic local anesthetic blocks of the infraorbital nerve (performed intraorally or extraorally) or the inferior alveolar nerve (performed intraorally) provide quick and effective relief.
10.2.G Trigger-Point Injections Using Local Anesthetics
Myofascial trigger points are well-known sources of referred pain in the orofacial and cervical region. The diagnostic value of trigger-point injections is when they are used to assess whether the trigger point in the muscle is responsible for the patient’s more distant pain complaint (referred pain). This assessment can be done in three ways. First, pain can be elicited by manual compression of the trigger point, which will often elicit not only focal pain at the trigger point site, but also distant pain in another area. Second, pain of the trigger point and sometimes at the referred sites can be suppressed briefly following stretching of the involved muscle.22 Third, trigger-point pain and usually the referred pain can be suppressed with a trigger-point injection. This is done by identifying a trigger point by palpation and then injecting it with 0.5 mL of 0.5% lidocaine without epinephrine. This provides prompt, symptomatic pain relief and helps to stretch the involved muscle.23
Trigger-point injections have both a therapeutic and a diagnostic value. This technique uses a small needle (usually 27 gauge), the syringes are Luer-lock disposable plastic syringes (either 1- or 3-mL size). The commonly used anesthetic solutions injected are 0.5% procaine and 0.5% lidocaine. Because procaine has reports of higher allergic reactions, the latter is usually preferred to reduce this risk.24 In addition to anesthetics, sometimes botulinum toxin A is used to treat resistant trigger points associated with taut bands. Most physicians and dentists use the anesthetic to provide some transient pain relief associated with immediate postinjection soreness and, more important, to ensure that any referred pain coming from a trigger point is suppressed as a result of the injection. It is unlikely that solutions stronger than 0.5% are more effective when injecting trigger points, and higher concentrations of these local anesthetic solutions increase the risk of myotoxicity.25 Of the anesthetic solutions, lidocaine is clearly more myotoxic than procaine. Epinephrine is never used with these injections as it is far more myotoxic than the anesthetic itself.26
Trigger-point injections have been described in the literature for more than 50 years.27 Limited data beyond open-label studies exists on the efficacy of this method of treatment. One study examined the relative efficacy of trigger-point injections within the context of a randomized double-blind protocol.28 The subjects were 63 low back pain patients and all had normal lumbosacral radiographs. They were assigned to one of four treatment procedures: (1) lidocaine, (2) lidocaine combined with a steroid, (3) acupuncture, and (4) vapocoolant spray with acupressure. The results indicated that an injection (with or without medication) was effective and that the injected substance was not critical to the effect. A systematic review of the myofascial trigger point literature concluded that direct needling of the trigger point was an effective treatment, but whether the effect is related to changes induced by needling the trigger point or nonspecific suppression of pain is not clear.29
10.2.H Topical Anesthetic Challenge Test in Neuropathic Pain Diagnosis
It is not uncommon to have a situation where a root canal is completed on a tooth and the patient still has pain. The typical diagnostic dilemma is to distinguish between a residual dental pulpal–periapical infection causing tooth pain and a sensitized alveolar nerve causing tooth pain. The latter is called a chronic trigeminal neuropathy or atypical odontalgia (AO). This condition is different from trigeminal neuralgia, which presents typically as episodic, sharp, shooting pain that lasts for a few seconds and occurs several times a day with pain-free intervals between attacks. Sometimes a peripheral nerve neuropathy will induce secondary central sensitization as well. This means that neural alterations extend into the trigeminal nucleus at the level of the pons, as well as in the third-order neuron and above.30–35 In these cases topical anesthetics may help establish that the pain is a neuropathic disorder. The best approach is to perform a local anesthetic challenge test (Table 10.1). This involves isolating the area, rating the pain, and then applying either a topical anesthetic or a nonanesthetic placebo to the painful site.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


