10 Lasers in Endodontics
Pulp Diagnosis (Laser Doppler Flowmetry)
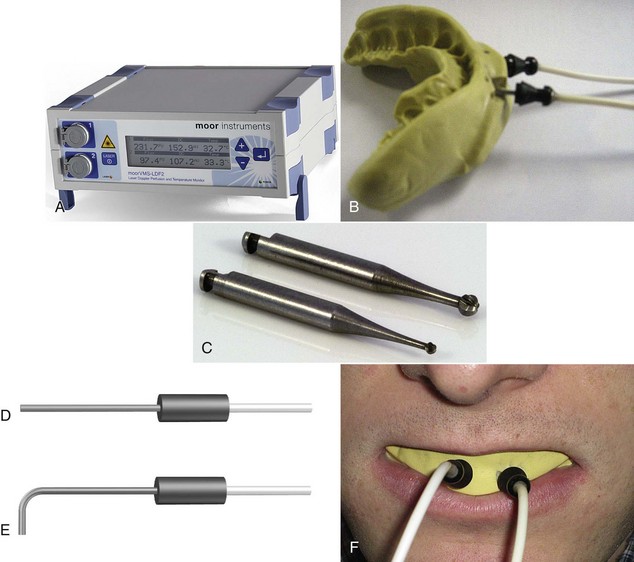
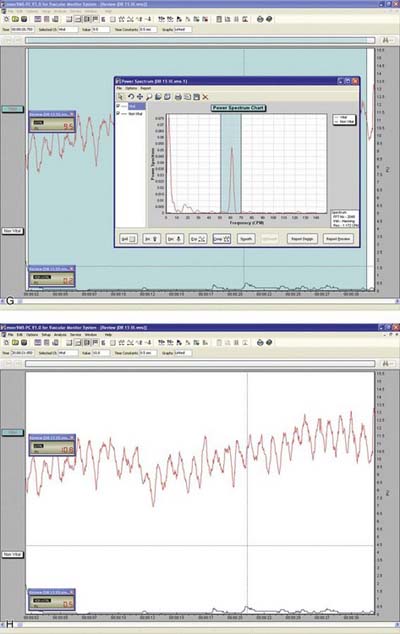
Laser Doppler flowmetry (LDF) was developed to assess blood flow in microvascular systems. It can also be used as a diagnostic system for measurements of blood flow in the dental pulp.1,2 LDF uses low-power settings (1-2 mW) of helium-neon (HeNe) or diode (810 nm) light sources.3,4 This technique may represent a sensitive and accurate means for pulp vitality testing because it reflects vascular rather than neural responsiveness compared with other methods.5 The laser beam must be directed through the clinical crown structure to the pulpal blood vessels, where flowing red blood cells (RBCs) cause the Doppler shifting of the frequency of the laser beam. Some of the light is backscattered out of the tooth and is detected by a photocell on the tooth surface. The output is proportional to the number and velocity of RBCs6,7 (Figure 10-1).
Interest in LDF has mostly been in the field of dental traumatology. Studies have found that LDF is effective in the assessment of revascularization of replanted immature dog teeth,8,9 as well as in patients with avulsed permanent maxillary incisors treated with replantation and splinting.4,10
Limitations in LDF mainly derive from environmental contamination, and therefore it may be difficult to obtain laser reflection from certain teeth.11 The anterior teeth, in which the enamel and dentin are thin, usually do not present a problem. Molars, however, with their thicker enamel and dentin and the variability in the position of the pulp within the tooth, may cause variations in pulpal blood flow.1,3 Also, differences in sensor output and inadequate calibration by the manufacturer may dictate the use of multiple probes for accurate assessment.12 Further, the probe design and bandwidth may affect the laser Doppler readings from vital and nonvital teeth.13 It has been suggested that up to 80% of the laser Doppler blood flow signal recovered from intact human teeth without a rubber dam in place is of nonpulpal origin, and that the role of the periodontium in some pulpal blood flow recordings has probably been underestimated.14 The use of rubber dam in combination with a rigid splint is recommended to enhance the validity of recordings.15
Frentzen et al.16 reported the following problems with LDF:
In traumatized teeth, where excitability of the pulp is reduced, LDF could present an acceptable alternative to conventional methods of vitality testing (thermal and electrical stimuli). Further investigations and technological improvements are still required. When equipment costs decrease and clinical application improves, this technology could be efficiently used for patients who have difficulty in communicating or for young children whose responses may not be reliable.2
Pulp Capping and Pulpotomy
Pulp capping, as defined by the American Association of Endodontists, is a procedure in which “a dental material is placed over an exposed or nearly exposed pulp to encourage the formation of irritation dentin at the site of injury.” Pulpotomy entails surgical removal of a small portion of vital pulp as a means of preserving the remaining coronal and radicular pulp tissues. Pulp capping is recommended when the exposure is small (≤1.0 mm17,18) and the patient is young. Pulpotomy is recommended when the young pulp is already exposed to caries and the roots are not yet fully formed (open apices).
The traditional pulp-capping agent is calcium hydroxide, or Ca(OH)2.19,20 When it is applied to pulp tissue, a necrotic layer is produced and a dentin bridge formed. The same may occur when the pulpotomy procedure is performed. A newer material, mineral trioxide aggregate (MTA), shows favorable results when applied to exposed pulp. It produces more dentinal bridging in a shorter time with significantly less inflammation. However, 3 to 4 hours is necessary for complete setting of the MTA.21–23 The success rate of pulp capping, whether direct or indirect, ranges from 44% to 97%. In pulpotomy, the same agents are used until root formation has been completed. Whether full root canal treatment should then be initiated is debatable.24,25
Since the introduction of lasers to dentistry, several studies have shown the effect of different laser devices on dentin and pulpal tissue. Whereas ruby lasers caused pulpal damage, Melcer et al.26 showed that the carbon dioxide (CO2) laser produced new mineralized dentin without cellular modification of pulpal tissue in beagles and primates. Shoji et al.27 applied CO2 laser to the exposed pulps of dogs, using a focused and defocused laser mode and a wide range of energy levels (3, 10, 30, and 60 W). Charring, coagulation necrosis, and degeneration of the odontoblastic layer occurred, although no damage was detected in the radicular portion of the pulp.
Jukic et al.28 used CO2 and neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers with an energy density of 4 J/cm2 and 6.3 J/cm2, respectively, on exposed pulp tissue. In both experimental groups, carbonization, necrosis, an inflammatory response, edema, and hemorrhage were observed in the pulp tissue. In some specimens a dentinal bridge was formed.
Moritz et al.29 used a CO2 laser in patients requiring direct pulp-capping treatment. An energy level of 1 W at 0.1-second exposure time with 1-second pulse intervals was applied until the exposed pulps were completely sealed. The pulps were then dressed with Ca(OH)2 (Kerr Life). In the control group, the pulps were capped with Ca(OH)2 only. Symptoms and vitality were examined after 1 week and monthly for 1 year; 89% of the experimental group had no symptoms and responded normally to vitality tests, versus only 68% of the control group.
In cases of deep and hypersensitive cavities, indirect pulp capping should be considered. A reduction in the permeability of the dentin, achieved by sealing the dentinal tubules, is paramount. Nd:YAG and 9.6-micron (µ) CO2 lasers can be used for this purpose. The 9.6-µ CO2 laser is well absorbed by the hydroxyapatite of enamel and dentin, causing tissue ablation, melting, and resolidification.30 The use of the 9.6-µ CO2 laser did not cause any noticeable damage to the pulpal tissue in dogs.31
White et al.32 found that use of a pulsed Nd:YAG laser with an energy level less than 1 W, with a 10-Hz repetition rate, and overall 10-second exposure time did not significantly elevate the intrapulpal temperature. According to results, these may be considered safe parameters because the remaining dentinal thickness in cavity preparations cannot be measured in vivo. It is therefore recommended that clinicians choose laser parameters lower than these safety limits.
Cleaning and Disinfecting the Root Canal System
Bacterial contamination of the root canal system is considered the principal etiological factor in the development of pulpal and periapical lesions.33–35 Creating a root canal system free of irritants is a major goal of root canal therapy, traditionally achieved through biomechanical instrumentation. Because of the complexity of the root canal system, however, complete elimination of debris resulting in a sterile root canal system is difficult.36,37 Also, a smear layer, which covers the instrumented walls of the root canal, is formed during this treatment.38–40
The smear layer consists of a superficial layer on the surface of the root canal wall approximately 1 to 2 µ thick and a deeper layer packed into the dentinal tubules to a depth of up to 40 µ.40 It contains inorganic and organic substances that include microorganisms and necrotic debris.41 In addition to possible infection of the smear layer itself, it can also protect the bacteria already present deeper in the dentinal tubules by preventing intracanal disinfection agents from penetrating into the tubules.42 Pashley43 states that a smear layer containing bacteria or bacterial products might provide a reservoir of irritants. Thus, complete removal of the smear layer would be consistent with the elimination of irritants from the root canal system.44
Also, Peters et al.45 clearly demonstrated that more than 35% of the canals’ surface area remained unchanged after instrumentation of the root canal using four different nickel-titanium (NiTi) preparation techniques. Because most current intracanal medicaments have a limited antibacterial spectrum and limited ability to diffuse into the dentinal tubules, newer treatment strategies designed to eliminate microorganisms from the root canal system should be considered. These must include agents that can penetrate the dentinal tubules and destroy the microorganisms located in an area beyond the host defense mechanisms, where they cannot be reached by systemically administered antibacterial agents.46
Numerous studies have also documented that CO2,47 Nd:YAG,47–49 argon,47,50 Er,Cr:YSGG,51 and Er:YAG52,53 laser irradiation has the ability to remove debris and the smear layer from the root canal walls after biomechanical instrumentation.
However, the intracanal use of lasers has several limitations.54 The emission of laser energy from the tip of the optical fiber or the laser tip is directed along the root canal and not necessarily laterally along the root canal walls.55 Thus, it is almost impossible to obtain uniform 360-degree coverage of the internal aspect of the root canal system surface using a laser.54,55 Also, because thermal damage to the periapical tissues is possible, safety must always be considered.55 Direct emission of laser irradiation from the tip of the optical fiber in the vicinity of the apical foramen of a tooth may result in transmission of the energy beyond the foramen. This in turn may adversely affect the supporting tissues of the tooth and can be hazardous in teeth close to the mental foramen or to the mandibular nerve.55,56
Matsumoto et al.3 emphasize the possible limitations of laser use in root canal systems, suggesting that “removal of smear layer and debris by laser is possible, however it is difficult to clean all root canal walls, because the laser is emitted straight ahead, making it almost impossible to irradiate the lateral canal walls.” They strongly recommend improving laser endodontic tips to enable irradiation of all areas of the root canal walls.
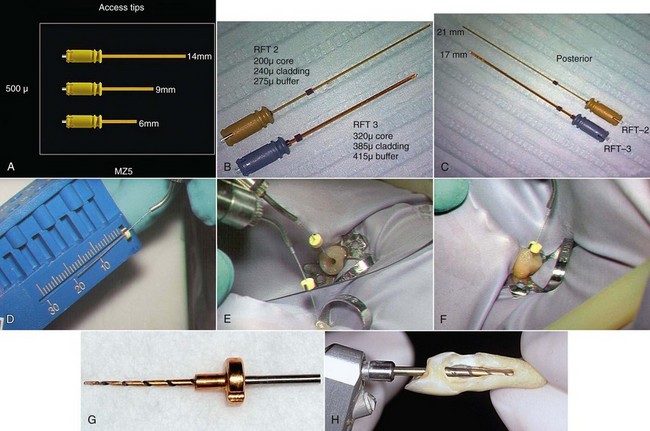
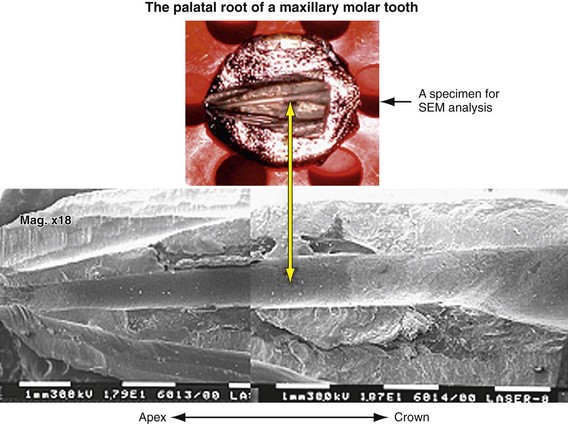
Erbium lasers have gained increasing popularity among clinicians after their clearance by the U.S. Food and Drug Administration (FDA) for use on hard dental tissues.57 Stabholz et al.55,56 describe a newer endodontic tip that can be used with an Erbium laser system. The beam of the Erbium laser is delivered through a hollow tube, permitting development of an endodontic tip that allows lateral emission of the irradiation (side-firing) rather than direct emission through a single opening at the far end. This new endodontic side-firing spiral tip was designed to fit the shape and the volume of root canals prepared by NiTi rotary instrumentation. It emits the Erbium laser irradiation laterally to the walls of the root canal through spiral slits located all along the length of the tip. The tip is sealed at its far end, preventing the transmission of irradiation to or through the apical foramen of the tooth. Examining the efficacy of the endodontic side-firing spiral tip in removing debris and smear layer from distal and palatal root canals of freshly extracted human molars, scanning electron microscopy (SEM) of the lased root canal walls revealed clean surfaces, free of smear layer and debris.56
The dentinal tubules in the root run a relatively straight course between the pulp and the periphery, in contrast to the typical S-shaped contours of the tubules in the tooth crown.41 Studies have shown that bacteria and their byproducts, present in infected root canals, may invade the dentinal tubules. Also, the presence of bacteria in the dentinal tubules of infected teeth was found at approximately half the distance between the root canal walls and the cementodentinal junction.58,59 These findings justify the rationale and need for developing effective means of removing the smear layer from root canal walls after biomechanical instrumentation. This would allow disinfectants and laser irradiation to reach and destroy microorganisms within the dentinal tubules.
In various laser systems used in dentistry, the emitted energy can be delivered into the root canal system by a thin optical fiber (Nd:YAG, KTP-Nd:YAG, Er:YSGG, argon, diode) or by a hollow tube (CO2, Er:YAG) (Figure 10-2). Thus, the potential bactericidal effect of laser irradiation can be effectively used for additional cleansing and disinfecting of the root canal system after biomechanical instrumentation. This effect was extensively studied using CO2,60,61 Nd:YAG,62–65 potassium titanyl phosphate (KTP)–Nd:YAG,66 excimer,67,68 diode,69 and Er:YAG70–72 lasers (Figures 10-3 to 10-5).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses