Chapter 2
Techniques for Oral Microbiology
Abstract
This chapter describes the techniques for oral microbiology, including smear and stain techniques, isolation, incubation and identification of oral microorganisms, various microscopy techniques, oral microecology techniques, and oral microbiome techniques.
Keywords
Biofilm; Incubation; Microscopy; Sequencing; Smear test; Stain test
2.1. Smear and Stain Techniques
Smear test and slide stain are basic techniques for microbial identification and are primarily used for morphological observation. The combination of smear and stain is widely used in oral microbiology research to differentiate between spirochetes, bacteria, fungi, and protozoa, as well as to identify specific cellular structures including spore, capsule, flagellum, and others. Currently, some of the commonly used techniques for cellular morphology examination are direct smear test and stained smear test, including Gram stain and Congo red stain. The procedure for smear test is shown in Figure 2.1.
Specific stains can be used to observe the specific microbial structures, including the bacterial spore, capsule, flagellum, fungal hypha, clamydospore, etc.
2.1.1. Kopeloff’s Modification of Gram Stain
Gram stain is a commonly used method for the identification of bacteria and fungi. After Gram staining, bacterial species can be generally differentiated into gram-positive and gram-negative groups, and most clinical anaerobic microbes can be differentiated by their typical cellular morphology. In addition, Mycoplasma with their ringshape can also be identified. Kopeloff’s modification of Gram stain is recommended by the Virginia Polytechnic Institute (VPI) for better visualization and differentiation of microbes.
2.1.1.1. Mechanism
Many theories have been proposed to explain the mechanism of Gram staining, including isoelectric point theory, chemical theory, and permeation theory. Among them, permeation theory has gained wide acceptance. It is believed that crystal violet placed on the microbe smear slides during staining interacts with aqueous iodine to form insoluble precipitates within the cell. During decolorization, alcohol and acetone can dissolve the lipid in the outer membrane and leach the precipitated dye–iodine complex out of the microbial cell. Gram-positive microbes have a thick cell wall with highly cross-linked peptidoglycans and relatively fewer lipids. Thus, gram-positive microbes cannot be easily decolorized and become deeply stained in purple. In contrast, gram-negative microbes have a thin membrane with high lipid content and little peptidoglycan. The precipitated dye–iodine complex can therefore be easily dissolved and leave the cell. As a result, the cell is stained red from the counterstain.
2.1.1.2. Preparation of Gram Stain Reagents (Commercially Available)
1. Crystal violet
Liquid A (add 10 g crystal violet to 1000 ml distilled water and mix well).
Liquid B (add 50 g NaHCO3 to 1000 ml distilled water and mix well).
2. Gram’s iodine
Dissolve 4 g NaOH into 25 ml distilled water and add 20 g iodine and 1 g KI. After everything is completely dissolved, add 975 ml distilled water, mix well, and store the liquid in a brown bottle.
3. Decolorizers
Add 300 ml acetone to 700 ml 95% alcohol and mix well.
4. Fuchsin counterstain
Add 5–10 g basic fuchsin to 100 ml 95% alcohol to form a supersaturated liquid. Mix 10 ml of the supersaturated liquid with 90 ml 5% carbolic acid solution followed by the addition of 900 ml water. Mix well and filter through filter paper. (Safranin solution can also be used in place of fuchsin solution for counterstain: add 20 g safranin to 100 ml 95% alcohol and adjust the volume to 1000 ml with distilled water.)
2.1.1.3. Staining Procedure
Gram stain procedure is shown in Figure 2.2.
1. Add crystal violet Liquid A onto the fixed smear slide. After 5 s, add Liquid B and gently shake the slide to mix Liquids A and B for 30 s. Gently rinse off the crystal violet with tap water.
2. Flood the smear slide with Gram’s iodine for 30 s. Gently rinse off the iodine with tap water.
3. Add decolorizer to the smear while holding the slide at an angle to allow the decolorizer to drain. Gently shake the slide for 5–10 s until crystal violet leaches out. Gently rinse off the decolorizer with tap water.
4. Flood the smear with fuchsin or safranin counterstain for 5–10 s. Gently rinse off any excess fuchsin or safranin with tap water. Drain and air dry the slide before observation.
2.1.1.4. Reporting Results
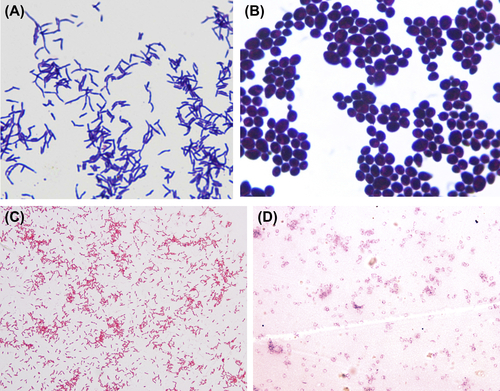
Examine the slide under a light microscope using oil immersion. Gram-positive bacteria appear purple (Figure 2.3(A) and (B)), while gram-negative bacteria appear pinkish red (Figure 2.3(C) and (D)).
2.1.1.5. Precautions
1. Smear only a monolayer of cells on the slide.
2. Avoid overheating of the smear slide.
3. Slightly prolong the time of decolorization if the smear layer is thick.
4. Old cultures of gram-positive bacteria may appear as gram-negative bacteria.
2.1.2. Staining Bacterial Spores
Gram stain and fuchsin-methylene blue are commonly used to observe bacterial spores.
2.1.2.1. Gram Stain
The Gram stain procedure is the same as described above. The result of Gram stain is shown in Figure 2.4.
2.1.2.2. Fuchsin-Methylene Blue Stain
2.1.2.2.1. Reagent Preparation
1. Fuchsin solution: Add 5–10 g basic fuchsin to 100 ml 95% alcohol to form a supersaturated liquid. Mix 10 ml of the supersaturated liquid with 90 ml 5% carbolic acid solution followed by 900 ml water.
2. Methylene blue solution:
Liquid A: Add 0.3 g methylene blue to 30 ml 95% alcohol.
Liquid B: Add 0.01 g KOH to 100 ml distilled water.
Mix Liquids A and B.
3. Decolorizer: 95% alcohol
Stain procedure: Flood the smear slide with fuchsin solution. Heat the slide on the alcohol burner to steam for 3–5 min (avoid drying out the smear slide and add fuchsin solution when necessary). Cool off the slide and rinse off excess fuchsin under tap water. Flood the slide with decolorizer for 1 min and rinse. Stain the slide with methylene blue solution for 0.5 min and rinse. Observe the slide under a microscope using oil immersion. Results of spore staining are illustrated in Figure 2.5.
2.1.3. Staining the Bacterial Capsule
The bacterial capsule can be stained using a variety of methods, including the Murs stain, ink methylene blue stain, Hiss copper sulfate stain, Ott safranin stain, etc. Encapsulated bacteria that are found in the oral cavity include Streptococcus pneumoniae, Porphyromonas gingivalis, and others.
2.1.3.1. Murs Stain
Preparation of staining solutions:
1. Staining solution (carbolfuchsin solution): Add 1 g basic fuchsin to 10 ml 95% ethanol solution, then add 40 ml 5% aqueous carbolic acid solution and mix.
2. Mordant staining solution: Mix 100 ml 2% tannic acid, 250 ml 10% potassalumite (K2SO4·12H2O), and 100 ml 7% saturated mercuric chloride.
3. Counterstaining solution (alkaline methylene blue solution): Add 0.3 g methylene blue to 30 ml 95% ethanol solution, then add 100 ml 0.01% potassium hydroxide solution.
4. Destaining solution: 95% ethanol.
Staining protocol: Prepare a smear sample using physiological saline and fix. Heated carbol fuchsin solution is used to stain the smear for 1 min. Flush with water after destaining using 95% ethanol. Stain with mordant staining solution for 0.5 min, and destain with 95% ethanol for 0.5 min. Stain with alkaline methylene blue solution for 0.5 min, then flush the stain solution with water. Examine microscopically after air drying. Typical results are shown in Figure 2.6.
2.1.3.2. Wright’s Stain
Bacterial capsules can also be stained with the Wright’s staining method. Furthermore, the Wright’s staining method can be used to stain Rickettsia and spirochetes, which appear purple after staining.
Preparation of staining solutions:
1. Wright stain solution: Weigh out 0.1 g dry Wright’s stain (same as eosin methylene blue stain). Grind eosin Y using mortar and pestle until it becomes a fine powder. Add 10 ml methanol to the mortar to fully dissolve the eosin Y. Add 20 ml methanol, mix, and allow to stand for a moment. Decant the liquid into a clean storage bottle, add methanol to the mortar, mix, and repeat several times until all of the stain is dissolved. Sixty milliliters of methanol should be used for 0.1 g stain. Shake and seal the storage bottle, and store it in the dark at room temperature.

Figure 2.4 Bacillus subtilis (Gram stain).
Under light microscope with oil immersion, bacterial spores appear colorless with strong refraction. They are located toward the center (central spore) or ends of the cell (terminal spore) with round or ovoid shape.

Figure 2.5 Spore stain with Clostridium tetani (fuchsin-methylene blue stain).
The cell appears blue and the spore appears red. A round spore is located at the end of the cell, giving the cell a drumstick appearance.
2. Phosphate buffer (pH 6.4–6.8): 30 ml 1% KH2PO4, 73.5 ml M/15 KH2PO4, 20 ml 1% Na2HPO4, 26.5 ml M/15 Na2PO4, complete with H2O to 1000 ml.
Staining protocol: Prepare a smear using the conventional method, and allow to air dry. Place four to six drops of Wright’s stain onto the smear and allow to stain for 1 min. Add an equal amount of phosphate buffer (pH 6.4–6.8). Shake gently to allow the phosphate buffer and the staining solution to mix evenly. Let the stain stand for 6–8 min and flush with water. The smear should appear pink (if the smear appears bluish violet, it can be destained by using 20% hydrochloric acid methanol solution). A typical result is shown in Figure 2.7.
2.1.4. Flagellar Stain
Flagellar stain is used to observe whether bacteria have flagella, as well as the number and location of flagella. It is a helpful tool for bacterial identification. Flagellar staining is of great importance in identifying motile oral bacteria. For example, Selenomonas can have up to 16 flagella, while straight Campylobacter only has one polar flagellum.
2.1.4.1. Preparation of Staining Solutions
Liquid A: 20 ml 2% tannic acid solution dissolved in heated water bath, 20 ml 20% potassium solution (heat to dissolve), and 50 ml 5% aqueous solution of carbolic acid. Mix all three solutions.

Figure 2.8 Periplasmic flagella (flagella staining).
The bacterial cell is stained red, and the light-red flagella can be seen around the bacterial cell.
Liquid B: alkaline fuchsin ethanol saturated liquid (same as in Gram stain solution). Mix 30 ml Liquid A with 10 ml Liquid B, and filter. Keep the filtrate for 6–10 h at room temperature for optimum results.
2.1.4.2. Smear Preparation
Flagellar staining requires correct preparation of the smear. Pick a small, young agar culture farthest away from the original streak, and resuspend in a small tube in sterile distilled water. Keep the small tube at room temperature for 20–30 min, or keep it in a 37 °C incubator for 4–5 min to allow the culture to become fully resuspended. Remove a loop of the bacterial suspension with a disposable sterile loop, and place it gently on a clean slide. Tilt the slide gently, or push the bacterial suspension with the loop. Then allow the smear to dry naturally at room temperature or in the 37 °C incubator.
2.1.4.3. Staining Protocol
Drop the Liquid A and Liquid B mixture onto a dry smear and allow to stain for 1–2 min. Flush the slide with water. When the smear is dry, observe the result under an oil immersion lens (Figure 2.8).
2.1.5. Staining Procedure for Special Fungal Structures
Gram stain and lactophenol cotton blue stain are used to visualize certain fungal structures that are significant for identification. These include the germinal tube, hyphae, and spores and are especially important in clinical analyses of oral mucosal diseases. For example, in the identification of thrush, angular stomatitis is closely related to C. albicans. The checking specimen is originated from albuginea or focal secretion of oral mucosa. Gram staining and acid phenol cotton blue stains are often used to verify the structures in fungi such as germ tubes, hyphae, and spores, especially for oral mucosal diseases such as thrush and angular cheilitis-related C. albicans. Most specimens are taken from the oral mucosa lesions albuginea and secretions.
2.1.5.1. Crystal Violet Stain
Crystal violet staining solution is prepared in the same way as Liquid A used in Gram stain. Take a small quantity of culture and mix with physiological saline to prepare a smear. Stain the smear with crystal violet solution. Observe under oil immersion lens (Figure 2.9(A) and (B)).
2.1.5.2. Lactophenol Cotton Blue Stain
Preparation of staining solution: Dissolve 20 g carbolic acid (solid), 20 ml lactic acid, and 40 ml glycerol into 20 ml distilled water (heat as gently as possible). Add 0.05 g cotton blue, shake until well mixed, and filter before storing.
Staining protocol: Place a drop of lactophenol cotton blue staining solution onto a clean slide, and mix the fungal culture or clinical sample with the staining solution. Place a coverslip on top and heat gently. Press the coverslip gently to remove any bubbles. Observe the slide under an oil immersion lens (Figure 2.10(A) and (B)).
Germinal tube and blastospores are stained bright blue after C. albicans are incubated in 0.5–1 ml human serum or in sheep serum for 2–4 h at 37 °C and stained with lactophenol cotton blue stain.
Pseudohypha chlamydospores of C. albicans are bright blue, and the big, spherical, thick-walled chlamydospore is at the tip or side wall of the pseudohypha.
2.1.6. Giemsa Stain for Mycoplasma
Giemsa stain is used to observe Mycoplasma (Figure 2.11).
2.1.7. Negative Congo Red Staining of Plaque Bacteria
Due to the simplicity of this method in producing high quality stained smears, the Congo red staining method has been widely used in the examination of periodontitis and other oral clinical specimens.
1. Sample: Saliva, plaque, other oral specimens.
2. Staining solution: 2% Congo red solution.
3. Staining procedure: Place a drop of the 2% Congo red solution on a clean slide. Mix the specimen and the staining solution, and spread the mixture thinly with a slide. After the smear dries naturally, smoke it over a bottle of concentrated hydrochloric acid until the red smear turns blue.

Figure 2.9 (A) Germinal tube and blastospore of Candida albicans (crystal violet stain). The germinal tube and blastospore appear purple when C. albicans is incubated in 0.5–1 ml human serum or in sheep serum for 2–4 h at 37 °C and stained by the crystal violet staining method. (B) Pseudohypha chlamydospore of C. albicans (crystal violet stain). Pseudohypha chlamydospores of C. albicans appear purple when stained with crystal violet. The chlamydospores are big, spherical, thick-walled, and they are located at the tip or side wall of unevenly stained pseudohypha.

Figure 2.10 (A) Germinal tube and blastospore of C. albicans (lactophenol cotton blue stain). (B) Pseudohypha chlamydospore of C. albicans (lactophenol cotton blue stain).
4. Results: The blue smear is examined under light microscope under oil immersion lens (Figure 2.12).
Listgarten classification is generally used in negative Congo red staining and dark-field microscopy to classify the observed plaque bacteria. The method involves selecting an evenly spread field, counting 200 bacterial cells, and reporting the percentage of different species according to their morphology.
Coccoid cells: Cell diameter of 0.5–1.0 μm, including several kinds of coccobacilli.
Straight rods: Cells measure approximately 0.5–1.5 μm in width, 1.0–1.9 μm in length. Some types of mycobacteria are included.
Filaments: Cells measure 0.5–1.5 μm in width, and the ratio of length to width is greater than 6. Most bacteria are shaped as irregular long filamentous cells.
Fusiform cells: Cells measure approximately 0.3–1.0 μm in diameter, 10 μm in length, and show tapered ends.
Curved rods: Cells are similar in dimension to straight rods, but are curved or crescent-shaped.
Spirochetes: Cells have a spiral shape and measure 0.2–0.5 μm in width and 10–20 μm in length.
2.1.8. Protozoan Smears
Entamoeba gingivalis and Trichomonas tenax are the main protozoans found in the oral cavity. Wet smears can be prepared using fresh specimens to observe the morphology and mobility of the protozoans. Giemsa stain can be used on a fixed specimen to observe the cellular structure.
2.1.8.1. Wet Smear for Fresh Samples
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses