Chapter 10
Periodontal Medicine Including Biopsy Techniques
Nodular proliferations on the gingiva are frequently encountered and represent a number of distinct entities with different etiologies and treatment strategies. While most represent reactive or inflammatory processes, occasionally lesions arise that are developmental in nature, perhaps resulting from stimulation of residua of odonotogenesis that persist in the oral mucosa following tooth development.
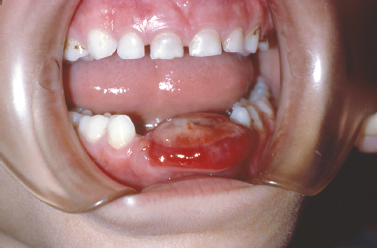
Inflammatory infiltrates at the apices of nonvital teeth occasionally channelize through medullary alveolar bone, penetrate the cortical bone and soft tissue, and drain into the oral cavity. These inflammatory infiltrates typically follow a path of least resistance. Given this trend, in most regions inflammatory apical lesions will drain into the oral cavity through a sinus tract on the buccal aspect of the alveolar bone due to decreased thickness of the buccal cortical plate compared with the lingual cortex. Exceptions to this rule are the mandibular second and third molars, the palatal roots of maxillary molars and the maxillary lateral incisors, which typically perforate lingually. At the orifice of the sinus tract, a focal nodular proliferation of inflamed granulation tissue may arise, termed a “parulis” or “gum boil” (Fig. 10.1).
Fig. 10.1. Nodular erythematous mass of granulation tissue near the mucobuccal fold and associated with an asymptomatic nonvital premolar. (Image courtesy of Dr. Helen Santis.)
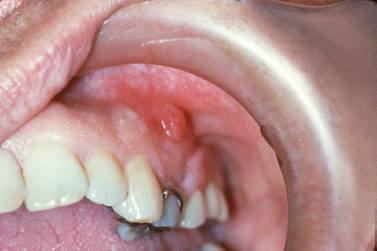
The parulis represents a focus of communication between a pathologic cavity associated with an odontogenic infection and the oral cavity. Therefore, it is frequently possible to insert a gutta-percha point into the sinus tract and trace its path to the tooth that represents the source of the infection. If the sinus tract remains patent, chronic drainage will allow the offending tooth to be asymptomatic. If the sinus tract becomes obstructed, symptoms of odontogenic infection will typically arise. A parulis typically resolves following endodontic therapy or extraction of the offending tooth; however, residual microorganisms and inflammation that persist along a sinus tract following treatment may cause the parulis to persist. In such instances, excision may be required.
A fibroma represents a nodular proliferation of dense fibrous connective tissue that arises secondary to trauma or focal irritation. Representing the most common reactive proliferation of the oral cavity, fibromas typically present as smooth-surfaced firm nodular lesions that are similar in color to the surrounding mucosa (Fig. 10.2). If the lesion is frequently traumatized or subjected to constant irritation, surface ulceration or hyperkeratosis may result. One form of fibroma with distinctive clinicopathologic characteristics termed the giant cell fibroma appears to have no association with trauma and is often described clinically as having a papillary surface architecture. With a predilection for occurring on the gingiva (Magnusson and Rasmusson 1995), the giant cell fibroma is typically diagnosed in patients under age 30. The histopatho-logic appearance is distinctive due to the presence of multi-nucleated and stellate cells throughout the densely collagenized connective tissue stroma thought to be derived from the fibroblast lineage (Souza et al. 2004); however, the presence of these stellate and multinucleated fibroblasts in this lesion is of no known clinical significance.
Fig. 10.2. Pink, smooth-surfaced nodular mass of the mandibular attached gingiva. (Image courtesy of Dr. Helen Santis.)
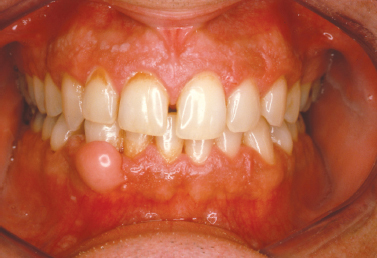
Representing a reactive nodular proliferation of fibrous and mineralized tissue, the peripheral ossifying fibroma is a frequently encountered lesion arising exclusively on the gingiva, most often from the region of the maxillary interdental papilla (Fig. 10.3). More common in females, the lesion typically presents in young patients anterior to the first molars (Cuisia and Brannon 2001) and may exhibit surface ulceration (Buchner and Hansen 1979). Although the pathogenesis is not completely understood, the peripheral ossifying fibroma is thought to represent a reactive process that frequently arises secondary to local irritation.
Fig. 10.3. Erythematous ulcerated mass of the palatal gingiva.
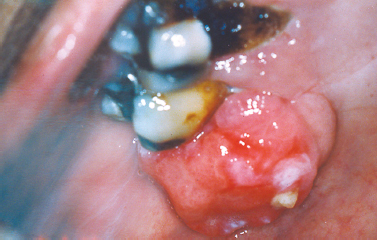
The pyogenic granuloma represents an acquired vascular lesion of the skin and mucous membranes that occurs in patients over a wide age range. Clinically presenting as a nodular lesion remarkable for rapid growth and frequently exhibiting surface ulceration, the pyogenic granuloma often bleeds on subtle provocation secondary to its vascular nature. Pyogenic granulomas of the oral cavity most commonly present on the gingiva in areas of focal chronic irritation (Fig. 10.4).
Fig. 10.4. Erythematous nodular mass arising from the mandibular anterior gingiva.
These lesions have been reported to occur with greater frequency in pregnant women. This increased incidence is likely related to increased levels of estrogen and progesterone, which have been shown to enhance angiogenesis in traumatized tissues (Yuan et al. 2002).
PERIPHERAL GIANT CELL GRANULOMA
Arising exclusively on the gingiva, the peripheral giant cell granuloma presents as an exophytic sessile or pedunculated nodular lesion that is often dark red or purple. Although seen over a wide age range, the peripheral giant cell granuloma typically presents during the fifth to sixth decades of life, is more commonly encountered in females, and is seen with greater frequency in the mandible anterior to the first molars (Bodner et al. 1997, Buduneli et al. 2001) (Fig. 10.5). Focal irritation is typically deemed the causative agent rather than a true neoplastic process. At least one study suggests diminished salivary flow rate and altered salivary composition may increase susceptibility to such lesions due to reduced ability to clear local irritants (Bodner et al. 1997). A pressure resorptive defect of the underlying bone may be appreciated in association with the peripheral giant cell granuloma having a “scooped-out” radiolucent appearance.
Fig. 10.5. Ulcerated reddish-purple mass of the mandibular anterior gingiva.
DIAGNOSIS AND TREATMENT OF REACTIVE GINGIVAL NODULES
Treatment of reactive gingival nodules, including the gingival fibroma, the peripheral ossifying fibroma, pyogenic granuloma, and peripheral giant cell granuloma, includes both a thorough excision of lesional tissue and removal of local irritants such as calculus or overextended restorations. Despite a diligent effort at complete excision, the recurrence rate for these lesions approaches 20% (Buduneli et al. 2001, Carrera Grano et al. 2001, Walters et al. 2001). To reduce the likelihood of recurrence, some suggest that reactive gingival lesions be excised to bone. In lesions recalcitrant to treatment, a wider excision including periosteum and curettage of the periodontal ligament may be indicated to prevent recurrence (Buduneli et al. 2001, Carrera Grano et al. 2001, Walters et al. 2001).
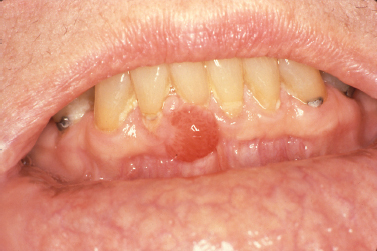
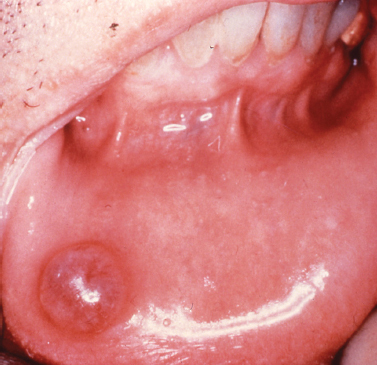
The gingival cyst of the adult represents an infrequently encountered lesion of odontogenic origin. Thought to originate from rests of dental lamina, the gingival cyst of the adult presents as a fluid-filled swelling typically arising on the labial attached gingiva of the premolar-canine region of the mandible (Buchner and Hansen 1979, Nxumalo and Shear 1992) (Fig. 10.6). Reported to primarily present during the fifth and sixth decades of life (Giunta 2002, Nxumalo and Shear 1992), the gingival cyst of the adult may occasionally cause a pressure resorptive defect of the subjacent alveolar bone that may cause the entity to be mistaken for a lateral periodontal cyst (Giunta 2002). Rare examples of multiple lesions have been described (Giunta 2002, Shade et al. 1987). Treatment consists of simple surgical excision with submission of lesional tissue for histopathologic examination.
Fig. 10.6. Fluid-filled lesion in the mandibular premolar region of the attached gingiva.
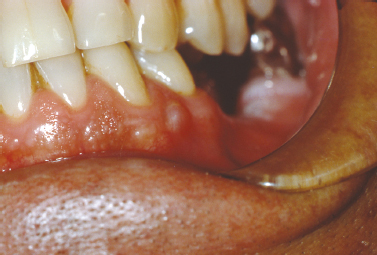
The mucocele is a frequently encountered lesion of the oral mucosa characterized by extravasated mucoid material that leads to a fluctuant nodular swelling of the mucosa, often remarkable for a bluish hue. Typically found on the lower labial mucosa lateral to the midline (Fig. 10.7) but noted in the buccal mucosa, floor of the mouth (ranula), and the anterior ventral tongue, mucoceles frequently arise secondary to a focal traumatic injury that causes rupture of an excretory duct with subsequent spillage of mucin into the surrounding connective tissue. The feeder salivary gland typically retains its capacity to produce secretion; however, the damaged duct prevents passage of saliva into the oral cavity. Patients typically complain of the lesion waxing and waning as the gland produces saliva and then empties. Occasionally, mucoceles are found on the posterior palate, posterior buccal mucosa, and retro-molar trigone; most frequently, these lesions represent superficial mucoceles. These lesions typically present as small fluid-filled vesicles that rupture, leaving ulcerative lesions that occasionally recur. Tarter control toothpaste (Navazesh 1995) has been linked to the formation of these lesions, and they have also been described to occur more frequently at mealtime.
Fig. 10.7. Bluish fluctuant swelling of the lower labial mucosa. (Image courtesy of Dr. Helen Santis.)
Additionally, superficial mucoceles have been reported in early stages of graft-versus-host disease (Garcia et al. 2002). A mucous cyst represents a true cystic lesion; the lining is derived from salivary ductal epithelium. These lesions are frequently located in the lips and buccal mucosa and are seen in association with ductal obstruction such as sialolithiasis that may increase intraluminal pressure; however, true developmental cystic lesions are seen.
Desquamative gingivitis is a clinically descriptive term that is characterized by sloughing, erythematous areas of the attached gingiva. These desquamative gingival changes may be appreciated in the context of vesiculoerosive conditions, including pemphigus vulgaris, mucous membrane (cicatricial) pemphigoid, erosive lichen planus, linear IgA disease, graft-versus-host disease, paraneoplastic pemphigus, epidermolysis bullosa acquisita, systemic lupus erythematosus, chronic ulcerative stomatitis, contact hypersensitivity reactions, and foreign-body gingivitis (2003) (Portions reprinted with permission from the Journal of the Massachusetts Dental Society 2005 Fall;54(3):38).
Lichen planus represents an immunologically mediated mucocutaneous disease that affects the oral cavity in as much as 2% of the population. Typically presenting in the fourth to fifth decades of life, lichen planus is characterized by a variety of clinical manifestations including the reticular, erosive, and atrophic forms of the disease. The most commonly affected site is the buccal mucosa, followed by the tongue, gingiva, labial mucosa and lower labial vermilion (Eisen 2002). In just under 10% of patients lichen planus presents exclusively on the gingiva (Eisen 2002, Mignogna et al. 2005) (Fig. 10.8). These cases typically present as the reticular or erosive forms of the disease involving wide areas of marginal and attached gingiva and most commonly affect women (Mignogna et al. 2005). A number of medications (especially antihypertensive agents), flavoring agents, oral hygiene products, and candies and chewing gum can elicit a mucosal reaction clinically indistinguishable from lichen planus. Termed lichenoid mucositis, clinical features, when correlated with histopathologic findings, help to distinguish the entity from bona fide lichen planus (Thornhill et al. 2006). Diagnosis is based on a process of elimination of suspected stimuli. This is frequently based on trial and error and is time consuming. If there is a reason to suspect medication-induced mucositis, consultation with the patient’s physician may be helpful to explore the possibility of substituting the medication. It is reasonable to treat this affliction with topical corticosteroids to control symptoms. Chronic ulcerative stomatitis represents an infrequently encountered condition that shares clinical features of erosive lichen planus superficially and may present as desquamative gingivitis. While the tongue and buccal mucosa are more commonly affected, gingival involvement is clinically indistinguishable from the erosive form of lichen planus (Solomon et al. 2003). This condition typically presents in women and is characterized by episodes of waxing and waning. Differences in the immunofluorescence profile aid in distinguishing chronic ulcerative stomatitis from lichen planus. The immunopathologic pattern for lichen planus is nonspecific but frequently consists of shaggy deposition of fibrinogen along the epithelial–connective tissue interface, whereas that of chronic ulcerative stomatitis consists of IgG autoantibodies directed against parabasal and basal stratified squamous epithelial cell nuclei. In some instances, a lichenoid appearance of the gingiva is seen secondary to the impregnation of dental materials into the gingiva during dental treatment. The term foreign body gingivitis is used to describe this entity. More common in females, foreign body gingivitis typically presents as an erythroplakic or erythroleukoplakic lesion of the gingiva (Fig. 10.9) that does not respond to improved oral hygiene measures or minimal improvement with topical steroid therapy as a result of its anti-inflammatory effect (Gordon 2000).
Fig. 10.8. Erosive lichen planus presenting as desquamative gingivitis with erythema and discomfort. (Image courtesy of Dr. Helen Santis.)
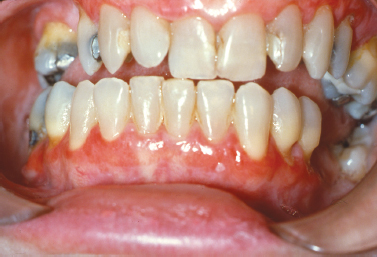
Fig. 10.9. Focal areas of painful erythematous marginal gingiva.
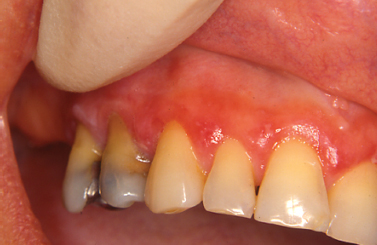
Pemphigus vulgaris represents an autoimmune-mediated mucocutaneous disease characterized by autoantibody attack against components of desmosomes. Frequently representing the initial manifestation of the disease, oral lesions are found in most instances and typically present as blisters or erosive lesions of the oral and pharyngeal mucosa. Often the onset is insidious with lesions getting progressively worse over time. Desquamative gingivitis in absence of other clinical features is a frequently encountered clinical presentation. Here, blisters and/or erosive lesions are seen often extending to the free gingival tissues (Mignogna et al. 2001) (Fig. 10.10). Direct immunofluorescence findings from tissue submitted in Michel’s media shows IgG or IgM antibodies and complement (typically C3) deposited in the intercellular areas of the epithelium. A condition linked to underlying lymphoproliferative disease termed paraneoplastic pemphigus may present with oral mucosal involvement yielding clinical characteristics indistinguishable from pemphigus vulgaris. In this condition, circulating autoantibodies produced in response to lymphoid neoplasia crossreact with antigens associated with epithelial desmoplakins and desmosomal proteins. Involvement of the oral mucosa is frequently seen and has been reported as the only manifestation of the disease (Bialy-Golan et al. 1996, Wakahara et al. 2005). Clinically, painful oral erosions and blister formation are appreciated on any mucosal surface including the labial vermilion. Skin eruptions are typically seen. Direct immunofluorescence findings for paraneo-plastic pemphigus show deposition of IgG and complement intercellularly within the epithelium and in a linear fashion along the basement membrane. These findings together with a history of lymphoproliferative disease and unique circulating autoantibody profile help to distinguish paraneoplastic pemphigus from other vesiculobullous disorders.
Fig. 10.10. Erosive lesions affecting the anterior mandibular gingiva and mandibular mucobuccal fold.
MUCOUS MEMBRANE (CICATRICIAL) PEMPHIGOID
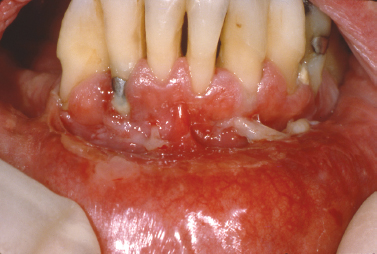
Mucous membrane (cicatricial) pemphigoid represents a group of immune-mediated mucocutaneous disorders in which autoantibodies are directed against basement membrane components. Although the disease typically affects patients in the fifth to sixth decades of life, rare examples of pemphigoid have presented in childhood; in some instances, lesions were limited exclusively to the gingiva (Cheng et al. 2001, Laskaris et al. 1988, Musa et al. 2002, Sklavounou and Laskaris 1990) (Fig. 10.11). Characterized by subepithelial separation from the underlying connective tissue, cicatricial pemphigoid presents as areas of erosive or vesiculobullous change throughout the oral mucosa with subsequent scarring. Ocular involvement and conjunctival scarring caused by the disease can lead to blindness. A subgroup of patients more severely affected by the disease appear to have both IgG and IgA circulating anti–basement membrane zone antibodies and frequently require systemic management (Setterfield et al. 1998). One condition clinically indistinguishable from cicatricial pemphigoid termed linear IgA disease is characterized by deposition of IgA along the basement membrane. The immunostaining profile is distinct from that of cicatricial pemphigoid, which is characterized by liner deposition of IgG (and occasionally IgM and IgA) and C3 along the basement membrane and is used to distinguish the two disease processes.
Fig. 10.11. Desquamative gingival lesions representing the only affected site in this patient. (Image courtesy of Dr. Helen Santis.)
DIAGNOSIS AND TREATMENT OF DESQUAMATIVE GINGIVAL LESIONS
Nonspecific inflammation frequently obscures critical features of underlying disease; therefore, it is recommended to avoid marginal gingiva when choosing a biopsy site for the diagnosis of mucosal fragility disorders. Prior to biopsy, verification of epithelial fragility should be sought by assessing the presence of the Nikolsky sign. Here, firm lateral pressure along the mucosal surface of clinically unremarkable tissue adjacent to involved mucosa will elicit bulla formation. Specimens representing the surface of a “de-roofed” vesicle may occasionally yield useful diagnostic information; therefore, any tissue obtained from clinical manipulation of the friable mucosa should be submitted for immunofluorescence analysis (Siegel and Anhalt 1993); however, a biopsy of perilesional tissue is recommended for diagnostic purposes. The specimen should be bisected with half and submitted in formalin for routine hematoxylin and eosin staining, and the other half should be submitted in immunofluorescence medium such as Michel’s for direct immunofluorescence. Immunofluorescence staining in conjunction with light microscopy is often required to make a definitive diagnosis of vesiculobullous disorders (Gallagher et al. 2005). Treatment of symptomatic lichen planus should begin after biopsy and histopathologic diagnosis. In mild cases, topical corticosteroids (0.05% fluocinonide gel [Lidex]) applied to the affected areas sparingly 4 times a day typically provides improvement in the symptoms and clinical appearance of the lesions within 4 weeks. For the most successful management, the patient is instructed to eat, brush, and then apply the gel with nothing per mouth for 30 minutes. It is important that the patient understands this is not a “cure” but rather an effort to maintain remission. More severe cases may require a brief course of systemic corticosteroid therapy in close consultation with the patient’s physician. Once the patient is in remission, the patient can be maintained with topical steroids. With the use of topical or systemic corticosteroids, it is not uncommon for patients to develop superimposed candidiasis. A 1-week course of fluconazole (Diflucan) 100-mg tablets (two tablets the first day and then one tablet each day following for 2 weeks) should provide relief in the absence of contraindications. In lesions consistent with foreign-body gingivitis, surgical excision of affected tissue is typically the requisite treatment approach. (Gravitis et al. 2005). In some instances, it is possible to identify the source of the foreign material using energy-dispersive x-ray microanalysis (Daley and Wysocki 1990, Gordon 1997, 2000). Chronic ulcerative stomatitis is frequently recalcitrant to topical steroid therapy and may require management with hydroxychloroquine (Plaquenil) 200 mg/day; however, systemic side effects, including irreversible retinopathy, neuromyopathy, agranulocytosis, aplastic anemia, and toxic psychosis (Solomon et al. 2003), associated with this medication necessitates consultation with a patient’s physician and close clinical follow-up. The management of pemphigus vulgaris and mucous membrane pemphigoid typically involves use of systemic corticosteroid therapy; however, a contemporary management approach involves treatment with Rituximab and intravenous immune globulin (Ahmed et al. 2006). It is necessary to have patients evaluated by a physician knowledgeable about this contemporary approach to management.
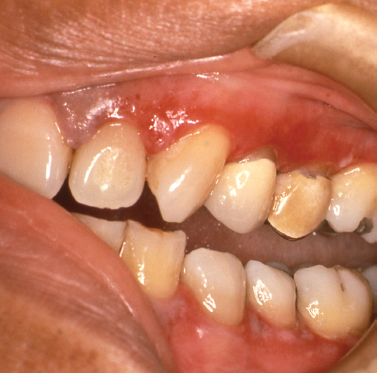
Plasma cell gingivitis represents a unique entity characterized by sharply demarcated erythema and enlargement of the free and attached gingiva (Fig. 10.12). This condition generally represents a hypersensitivity reaction to flavoring agents in oral hygiene products, candies or chewing gum, medications, or a component of the diet (Macleod and Ellis 1989, Serio et al. 1991). Despite this fact, in many instances the offending antigen cannot be isolated. Biopsies of such lesions show a dense infiltrate of plasma cells within the connective tissue stroma subjacent to the epithelium. Because a neo-plastic plasma cell proliferation cannot always be excluded on light microscopic examination alone, additional studies to determine the nature of the infiltrate may be indicated. In cases of idiopathic plasma cell gingivitis or cases representing a hypersensitivity reaction, the plasma cell infiltrate is polyclonal and does not show an atypical profile on immuno-electrophoresis. When other oral mucosal sites are involved, the condition is termed plasma cell mucositis. Here, diffuse, erythematous, and edematous changes may involve multiple areas of the oral mucosa (Heinemann et al. 2006, Kaur et al. 2001). Treatment of plasma cell gingivomucositis requires that the patient record all food intake and eliminate possible dietary culprits. Additionally, discontinuance of use of chewing gums, candies, and/oral hygiene products remarkable for strong flavoring agents such as peppermint or cinnamon should be encouraged. Unfortunately, in some instances the underlying causative agent cannot be identified. Topical steroid agents (fluocinonide 0.05% gel [Lidex]) applied to the affected areas sparingly 4 times a day may provide some improvement. This regimen is most effective if the patient eats, brushes, and then applies the gel to the affected areas with nothing per mouth for 30 minutes following application. It is generally recommended that follow up evaluation after 4 weeks of topical corticosteroid application be done and the frequency of use adjusted until improvement is optimal.
Fig. 10.12. Diffuse, erythematous changes of the attached and free gingival tissues.
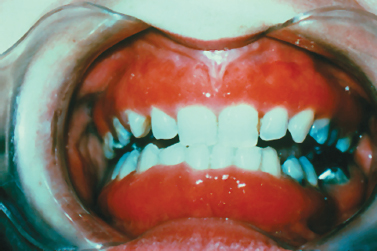
Representing an acute hypersensitivity reaction involving the skin and the mucosa, erythema multiforme (EM) most typically presents in the third and fourth decades of life; however, a significant number of patients diagnosed with EM are children (Huff et al. 1983, Wine et al. 2006). Although the pathogenesis is poorly understood, the condition is likely an immune-mediated disorder. EM is induced by a variety of factors, of which the most common include bacterial and viral infectious agents and medications, most typically analgesics and antibiotics. Approximately 50% of cases arise subsequent to infection with herpes simplex virus. Two forms of EM are typically described: erythema multiforme major and erythema multi-forme minor. EM minor presents with lesions involving the skin and/oral mucosa. It is often recurrent, with the most frequent cause of recurrent EM being a herpes simplex virus infection (Huff et al. 1983, Sinha et al. 2006). EM major is also referred to as Stevens Johnson syndrome and is typically triggered by mycobacterium and medications (Huff et al. 1983). Here, in addition to cutaneous and/oral lesions, ocular and/or genital mucosae are affected. Symblephara or bands of scar tissue within the conjunctiva can lead to blindness akin to ocular lesions of cicatricial pemphigoid. The most severe form of EM termed toxic epidermal necrolysis (Lyell’s disease) involves sloughing and ulceration of large areas of the skin and mucosa. Mucosal lesions of EM are characterized by painful ulcerative lesions of acute onset (Fig. 10.13), often involving the labial mucosa with crusting of the vermilion. Gingival involvement is rare but is occasionally reported. Classic skin eruptions are described as “target lesions” and are seen in approximately 25% of all patients presenting with EM (Ayangco and Rogers 2003). Here, concentric erythematous rings likened in appearance to a target or “bull’s eye” are appreciated on the extremities initially and occasionally extending to involve other cutaneous sites. Unlike other vesiculobullous disorders, the attached mucosae including that of the gingiva and hard palate are typically unaffected by the process. In most instances, EM resolves spontaneously over the course of a 2- to 4-week period. Treatment is typically supportive and directed at managing symptoms. In severe cases one may consider systemic corticosteroid therapy. Here, prednisone tablets (10 mg) may be prescribed with the instruction to take 6 tablets in the morning until the lesions recede and then decrease by 1 tablet on each successive day (Arm et al. 2001). Dexamethasone (Decadron) elixir 0.5 mg/5 mL can also be used. Here, one can recommend the patient rinse with 1 tablespoonful (15 mL) for 3 days 4 times per day and swallow. Then, for 3 days, rinse with 1 teaspoonful (5 mL) 4 times a day and swallow. Then, for 3 days, rinse with 1 teaspoonful (5 mL) 4 times a day and swallow every other time. Last, the patient should rinse with 1 teaspoonful (5 mL) 4 times per day and expectorate (Arm et al. 2001). If recurrent EM is thought to be precipitated by herpes simplex virus, antiviral medications are typically prescribed such as systemic acyclovir (Zovirax) 400 mg capsules administering 1 tablet 3 times daily or valacyclovir (Valtrex) 500-mg capsules administering 1 tablet per day.
Fig. 10.13. Ulceration and crusting lesions involving the labial vermilion.
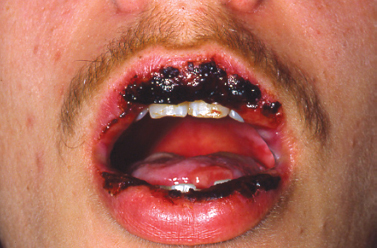
Gingival enlargement may be focal or diffuse in nature. Focal gingival enlargement is seen in association with a number of entities ranging from benign reactive proliferations to malignant epithelial neoplasia. Generalized gingival enlargement is likewise seen in association with a variety of conditions. Systemic medications, neoplastic infiltration, infection, and hereditary conditions may all present with generalized gingival enlargement. The diagnosis and treatment of such lesions are discussed later.
Epulis fissuratum is characterized by folds of hyperplastic fibrovascular connective tissue that develop in association with an ill-fitting denture. These redundant folds of tissue frequently extend into the vestibule with invaginations to accommodate the denture flange (Fig. 10.14). Treatment consists of surgical excision to ensure improved soft tissue contour for impression making and fabrication of a new prosthesis.
Fig. 10.14. Redundant hyperplastic folds of tissue in the anterior maxillary associated with a maxillary denture.
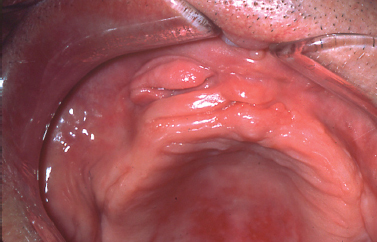
MEDICATION-INDUCED GINGIVAL OVERGROWTH
Numerous medications have been implicated as the causative agent for diffuse gingival enlargement. The medications most commonly associated with gingival overgrowth include calcium channel blockers (Fig. 10.15), cyclosporine, and anticonvulsant medications. Although incompletely understood, it seems such medications target a common pathway of collagen degradation; interference with this pathway induces fibrosis and extracellular matrix overgrowth in the gingival tissues (Kataoka et al. 2005, McCulloch 2004). Introduction of the causative medication in childhood seems to increase the likelihood of occurrence. The most typical clinical course for the process begins as diffuse gingival enlargement of the facial surfaces of the gingiva most prominently along the interdental papillae several weeks following the initiation of a medication. Over time, the tissue overgrowth extends to the lingual surfaces of the gingiva and can completely cover the dentition. Typically, hyperplastic changes are not appreciated in edentulous areas unless the tissues approximate poorly fitting pros-theses or surround dental implants. Oral hygiene dictates the clinical appearance of the hyperplastic gingival tissues. In patients with adequate oral hygiene, the hyperplastic tissues maintain a pink color and stippled appearance. In patients with marginal oral hygiene, the tissues may become friable and bleed on subtle provocation.
Fig. 10.15. Marked gingival hyperplasia in a patient using calcium channel blocker agents.
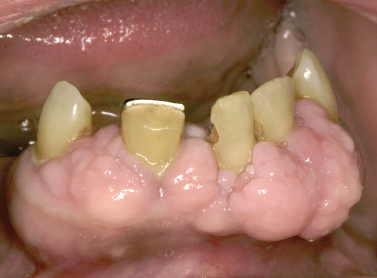
Treatment for medication-induced gingival hyperplasia includes substitution of the inciting medication with a different agent that is less likely to cause gingival hyperplasia when possible and encouraging meticulous oral hygiene. Additionally, supplementation with folic acid may reduce the incidence of medication-induced gingival overgrowth; however, the results of such efforts have been mixed (Ba/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


