Periodontitis is a complex inflammatory human disease. According to the World Health Organization (WHO), periodontitis is considered as a chronic noncommunicable disease (NCD), and it shares social determinants and risk factors with other NCDs such as cardiovascular diseases, diabetes mellitus, cancer, and chronic pulmonary diseases, which account for two-thirds of the worldwide mortality.1 Thus, prevention of periodontal diseases, such as gingivitis and periodontitis, is highly important for each individuum.
This chapter will give an overview on the current knowledge of the epidemiology, etiopathogenesis, diagnosis, and preventive as well as therapeutic considerations in relation to periodontitis.
Periodontitis is one of the most prevalent oral diseases that can be prevented or treated. Figure 2-1 displays a representative image of a patient with periodontitis. In this specific case, the patient experienced tooth movement, tooth mobility, and generalized bleeding on probing. In addition to the resulting gaps between teeth, plaque deposits and discolored swollen gingival tissue are clearly visible in the interdental regions.

Fig 2-1 Representative image of a patient with periodontitis. This image shows the sequelae of periodontal destruction due to periodontitis: tooth movement, tooth mobility, and generalized gingival inflammation. In addition to the resulting gaps between teeth, plaque deposits and discolored swollen gingival tissue are clearly visible in the interdental regions.
Gingivitis and periodontitis are considered as two entities of the same biofilm-induced inflammatory disease that affects tissues around teeth.2 While gingivitis is a reversible inflammation of the gingiva around teeth without attachment loss, periodontitis comprises an additional nonreversible degradation of the periodontal apparatus, including the alveolar bone. In the worldwide population, approximately 12% exhibit severe forms of periodontitis, whereas approximately 50% show mild to moderate forms of periodontal disease. It is important to note that periodontitis represents the sixth most common human disease.3,4 According to the increasing world population and high numbers of retained teeth, the global burden due to severe periodontitis increased between 1990 and 2013 by 67%.5 This led to a higher economic load to the health care systems.6
With respect to the distribution of periodontal disease in humans, periodontitis is an age-associated but not age-dependent disease, and the prevalence of periodontitis is strongly elevated between the third and fourth decade of life.3,7 Low socioeconomic status is associated with higher risk for periodontitis, and men suffer more from periodontitis compared to women.8,9
Etiopathogenesis of periodontitis
The periodontium is a unique structure in the human body. While body surfaces, such as skin, mucosal tissues, hair, and nails, are constantly renewed, shed, and grow out, respectively, teeth exhibit a nonshedding surface. In addition, the tooth represents a direct connection between bone and the outside microbial environment, only separated by the junctional epithelium and connective tissue fibers. This allows for the dental plaque biofilm to develop and grow along the tooth surface into the gingival crevice, if not disturbed by routine oral hygiene procedures.
Microbial factors
Although periodontitis is a multifactorial disease, the central role to its pathogenesis refers to the interaction between the dental plaque biofilm, mainly consisting of well-organized bacteria adhering to the dental surface, and the immune inflammatory reaction of the host.10
In this context, periodontal health represents a status of homeostasis associated with a symbiotic biofilm and an appropriate immune-inflammatory reaction of the host, including the presence of neutrophils and the expression of antimicrobial peptides.11,12
The immune response to the periodontal bacteria is different for each individuum and impairs the composition of the bacterial biofilm differentiating from a symbiotic into a dysbiotic composition.13 The change of the relative abundance of pathogenic microorganisms when compared to their abundance in a healthy state leads to alterations within the host-microbial interactions that can further accelerate the inflammatory responses.13
The increasing amount of pathogenic periodontal bacteria, including their virulence factors, leads to an elevation of cellular signal transduction, and subsequently, secretion of pro-inflammatory mediators by various periodontal cell types.14–16 Furthermore, the shift within the microbiota activates the complement system.17 In the course of periodontal inflammation, more immune competent cells, such as macrophages, polymorphonuclear neutrophil granulocytes, T-cells, and B-cells, are present. The synthesis of additional pro-inflammatory mediators, such as interleukins, cell stimulating and receptor activating factors, and proteinases leads to changes of the connective tissue and bone metabolism, and therewith, to periodontal attachment loss.18
Initially, a dysbiosis between an altered biofilm in combination with a dysregulation of the immune reaction of a susceptible host leads to an inflammation of the gingiva (Fig 2-1). Gingivitis is a reversible disease that affects epithelium and connective tissue. The ongoing imbalance between the dysbiotic biofilm and the host-related immune reaction can lead to degradation of connective tissue and alveolar bone.10,12,19–22
In the context of the etiopathogenesis of periodontitis, the role of periodontal bacteria has been investigated for decades, and different plaque hypotheses have been developed.23 Several oral bacterial species, eg Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, have been regarded to be pathogenic. Within the periodontal microbiota, a special role is awarded to P gingivalis, a Gram-negative, anaerobic periodontal bacterium. P gingivalis is considered to be a keystone pathogen in the etiopathogenesis of periodontitis. According to its specifications, P gingivalis can influence the course of the periodontal disease by remodeling a health-associated homeostatic biofilm composition into a dysbiotic biofilm composition, even at very low levels of colonization (< 0.01 % of the bacterial count in an animal model).13,24
Within the framework of inflammatory reaction, intercellular connections of periodontal cells disintegrate, and bacteria and their metabolic products can invade into deeper tissue levels, enter the bloodstream, and, eventually, reach the tissues beyond the oral cavity.25,26 Throughout the body, periodontal bacteria may activate immune cells, which then release pro-inflammatory mediators. In this context, periodontal bacteria are able to modify immune-inflammatory reactions in the whole body. Therefore, it seems to be conceivable that periodontitis may also affect other inflammation-driven diseases, such as diabetes mellitus, cardiovascular diseases, and rheumatoid arthritis (see below).
Smoking
Smoking is considered to be one of the most important modifiable risk factors for periodontitis, with a clearly documented dose-response relationship.27–29 In this context, smoking affects the composition of the microorganisms within the dental plaque biofilm leading to a higher proportion of pathogenic periodontal bacteria. Moreover, smoking impairs microcirculation and immune response to the bacteria, such as disturbance of the neutrophil function, increased synthesis of pro-inflammatory mediators, and higher levels of activated T-cells.30,31
In the context of periodontitis, the progression of periodontal break down is highly increased in smokers when compared to nonsmoking individuals with periodontitis.32 The importance of the smoking status has now been integrated into the classification system of periodontal diseases.33
In respect to the progression of periodontitis, smoking represents one of the two modifying factors affecting the grading of periodontitis.33 Smoking cessation has positive effects on periodontal conditions, the treatment of periodontitis, and tooth retention.34–38
Nutrition
Nutrition and malnutrition, respectively, seem to be of importance in relation to periodontal diseases. It is known that nutrient factors influence inflammatory reactions, and malnutrition may lead to depletion of micronutrients which then increases the susceptibility to periodontitis.39 For example, there is an association between periodontal inflammatory responses and vitamin C depletion. Historically, the relationship between the intake of vitamin C-rich vegetables or fruits and periodontal disease has been described. In the 18th century, during maritime trading and exploratory seafaring, sailors often suffered from a condition called scurvy. Scurvy represents a vitamin C deficiency disease associated with gingival bleeding and tooth mobility caused by altered collagen formation, impaired connective tissue barrier formation, and fibroblast growth.40–42 There are a number of different micronutrients associated with periodontal health and disease. For macronutrients, it has been observed that a higher intake of sugar is associated with increased gingival bleeding.43,44 For more detailed information, the authors refer to the relevant literature.39
Obesity
The association between obesity and periodontitis has been described more recently. It was found that central adiposity was associated with an increased risk of developing periodontitis in older adults. However, due to large variability within the available studies, only moderate evidence is currently available.45,46 In addition, there are some indications that periodontal therapy is more ineffective in obese patients with periodontitis. The effect on the therapeutic outcome was similar to the effect of smoking in periodontitis patients.47
In basic science experiments, it has been demonstrated that the differentiation of osteoblasts was altered in the presence of P gingivalis in obese mice.48 Animal experiments have also shown that age and obesity represent risk factors that may result in reduced alveolar bone crest height even in an otherwise healthy periodontium.49 Future research is required in order to gain more robust knowledge of how nutrition is related to periodontitis.
Genetic factors
The contribution of genetics to the risk for periodontitis has been estimated as up to 50%. In particular, a strong genetic component is assumed in younger patients with severe periodontitis.50
For several years, the identification of single nucleotide polymorphisms (SNPs) in relation to periodontitis was of high scientific interest.51 SNPs represent the most frequent genetic variation, and an association of this type of variation with disease is, therefore, very likely. Mostly, candidate gene studies have been performed in the past. The evidence, however, for one of the analyzed variations in candidate genes, such as for interleukin-1, is rather weak.52,53 In relation to periodontitis, it is very likely that genetic susceptibility factors for periodontitis will not be found in the genetic sequence of one distinct candidate gene, but rather in a variety of different genes. The technical progress in combination with the publication of the last human chromosome as part of the human genome project (sequencing of the whole human genome) allowed the simultaneous analysis of high numbers of genetic variations (SNPs), with no distinct a priori hypothesis.54 Those genome-wide association studies (GWAS) have been performed with large sets of samples from patients with periodontitis.55–58 To date, a number of different genetic variances in specific genes, such as ANRIL, PLA, SIGLEC5, DEFBA1A3, NPY, and GLT6D1 have been described in periodontitis patients, underlying the hypothesis that there are polygenetic factors that, in a certain but yet unknown combination, alter the susceptibility for periodontitis.57–61 The individual functional characteristics of the identified variations are the subject of current research projects.62
Periodontitis in relation to systemic diseases
Diabetes mellitus
Diabetes mellitus is a metabolic condition with chronic hyperglycemia as the leading symptom. Both diabetes mellitus and periodontitis are chronic, inflammation-driven diseases with mutual influence regarding their impaired immunologic responses.63,64
Diabetes mellitus as well as periodontitis are common, chronic, noncommunicable diseases that exhibit a bidirectional relationship (review65). The relationship between both diseases was first described by Löe in 1993, and periodontitis found as the sixth complication of diabetes mellitus.66 It has been shown that patients with diabetes mellitus exhibit more severe and rapidly progressive forms of periodontitis compared to patients without diabetes mellitus.67–70 It is now widely accepted that diabetes mellitus represents a major risk factor for the development and progression of periodontitis, and thus, diabetes mellitus has now been added as a modifying factor within the grading of periodontitis.29,33
Furthermore, during severe periodontitis, the inflammatory process can have detrimental effects on blood sugar control by influencing the insulin resistance, leading to a poorer glycemic control, increasing the rate of incident prediabetes and type 2 diabetes mellitus.71–74 Here, the periodontal inflammation exhibits a systemic impact along with bacteremia and increased levels of pro-inflammatory mediators detectable in the blood stream, and therewith, blood sugar control and insulin resistance are negatively influenced in patients with periodontitis and diabetes mellitus.65,70,75–77
Diabetes mellitus-related complications, such as cardiovascular, cerebrovascular, renal, retinal, and neuropathic complications, are significantly increased in patients with severe periodontitis and type 2 diabetes mellitus.78–82
Cardiovascular diseases
Cardiovascular diseases also belong to the group of chronic noncommunicable diseases, and it has been shown that there is a clear epidemiologic association with periodontitis.83,84 More precisely, epidemiologic studies have demonstrated that there is a positive association between periodontitis and coronary heart and cerebrovascular disease.84,85
Similar to diabetes mellitus, one of the proposed mechanisms of interaction includes the incidence of bacteremia due to daily routine procedures such as tooth brushing or eating.25 It was found that the magnitude of bacteremia was higher in patients with periodontitis when compared to patients with gingivitis.26 Analyses of atherothrombotic tissues revealed the presence of periodontal bacteria, and patients with periodontitis showed a greater probability for the detection of periodontal pathogenic bacteria in those tissues.86,87
In this context, the influence of periodontitis on the systemic inflammatory status has been recognized as an important mechanism.75,76 When compared to healthy control individuals, patients with periodontitis exhibited elevated levels for C-reactive protein (CRP), interleukin-(IL)-1 beta, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α).88–96
Dyslipidemia is recognized as a risk factor for cardiovascular diseases, and it has been shown that dyslipidemia is present in patients with periodontitis.97,98 Other parameters, such as increased intima media-thickness or alterations in the flow-mediated dilatation of blood vessels, may indicate pathologic cardiovascular alterations. Periodontitis is associated with an increased intima media-thickness, changes in the flow-mediated dilatation, and hypertension.99–103
It is an interesting finding that both periodontitis and cardiovascular diseases seem to share common genetic risk factors.58,59,104 This functional and molecular background is the subject of current research projects.62
Rheumatoid arthritis
Rheumatoid arthritis is a complex autoimmune disease that is characterized by the infiltration of macrophages and T-cells into the synovial membrane of joints leading to cartilage degradation and bone erosion.105 The production of auto-antibodies is typical for rheumatoid arthritis, and the auto-antibody against immune globulin G represents the rheumatoid factor.106,107 Biochemical post-translational processes, such as citrullination and carbamylation, are involved in the mechanisms leading to antibody synthesis.108,109 Citrullination is a post-translational modification of the amino acid arginine to citrulline promoted by an enzyme called peptidylarginine deiminase (PAD). Based on the current knowledge, the link between rheumatoid arthritis and periodontitis could be via the periodontal pathogen P gingivalis, which also expresses a similar enzyme (PPAD).110 The proteins modified by the PPAD from P gingivalis can further promote autoantibody production against citrullinated proteins (anti-citrullinated protein antibodies [ACPAs]), and this may be another stimulus for the development of rheumatoid arthritis.108,110 Current research investigates the hypothesis of periodontitis to be a risk factor for rheumatoid arthritis.111,112
Periodontitis may exhibit a considerable influence on systemic conditions such as diabetes mellitus, cardiovascular disease, and rheumatoid arthritis.
Clinical features and diagnosis
Symptoms, clinical and radiographic features
Periodontitis represents major clinical characteristics including microbial biofilm formation (dental plaque biofilm), inflammation of the gingiva, periodontal attachment loss, and bone loss. The microbial biofilm is visible as dental plaque on tooth surfaces and around the gingival margin, which may calcify and form dental calculus when not routinely removed by daily oral hygiene procedures. In most patients, the amount of plaque formation and calculus is associated with severity of periodontitis. Inflammation of the gingiva around the teeth can be seen by swelling, redness, edema, and bleeding. Pocket formation, bleeding on probing, and gingival recessions combined with periodontal attachment and bone loss are clinical signs of periodontitis. Furthermore, patients with severe periodontitis often suffer from tooth migration, increased tooth mobility, and periodontal abscesses. Clinical periodontal examination comprises the measurements of attachment level, periodontal pocket probing, bleeding on probing, furcation involvement, and tooth mobility. The amount and the type of bone loss (horizontally versus vertically), furcation involvement, and combined periodontal-endodontic lesions are determined by additional radiographic examinations. Figures 2-2 to 2-11 display the periodontal screening index, different types of periodontal explorers, and a complete patient case documentation during active periodontal therapy, and further background information is given in the corresponding legends.



Figs 2-2a to c Periodontal Screening Index (PSI). (a) Periodontal conditions are explored by a periodontal probe proposed by the World Health Organization (WHO). This probe is graded in sections of 0.5 mm, 3.5 mm, and 5.5 mm, respectively. Depending on the depth of penetration, different codes refer to the indication of health or disease. Shallow pockets (< 3.5 mm) without bleeding upon probing are considered healthy (code 0) and with bleeding on probing indicate gingivitis (code 1 and code 2 when calculus and/or overhanging restoration margins are present), whereas pockets > 3.5 mm to 5.5 mm indicate moderate, and ≥ 5.5 mm indicate more severe periodontal destruction (when no pseudopocket is present). (b) These codes are charged to each of the six quadrants; * indicates special findings, such as recessions, furcation involvement, tooth mobility, and migration. (c) The PSI allows a quick and safe examination of periodontal condition in the dentition. It helps to clearly distinguish between health and more or less severe disease without claiming a distinct periodontal diagnosis.



Figs 2-3a to c For a complete periodontal examination, more precisely scaled periodontal probes are required. (a) The PCPUNC 15 explorer (pressure calibrated, diameter of 0.5 mm) represents the standard periodontal probe with a graduation of 1 mm steps. (b) To explore interradicular attachment loss, a bended periodontal probe (Nabers probe, 3 mm graduation) is recommended for determination of furcation involvement. (c) Presentation of the application of periodontal probes around molar teeth.






Figs 2-4a to f A 38-year-old (at time of admission) man with an inconspicuous general health and no medication. Diagnosis: periodontitis stage 4, grade C. This case continues in Fig 2-10. Baseline examinations: (a) first and fourth quadrants; (b) second and third quadrants; (c) frontal view; (d) maxillary arch; (e) mandibular arch; (f) baseline measurements of the clinical attachment level, including tooth migration and loosening, furcation involvement, and bleeding on probing (graphic illustration by Parostatus.de).


Figs 2-5a and b Radiographic examination of the patient introduced in Fig 2-3. (a) Panoramic radiograph and (b) intraoral radiographs. The radiographs show severe horizontal and vertical (locally) bone loss.

Fig 2-6 Periodontal measurement of the clinical attachment level, including tooth migration and mobility, furcation involvement, and bleeding on probing at the time of reevaluation (3 months after nonsurgical debridement; graphic illustration by Parostatus.de).





Figs 2-7a to e Representative images of the corrective therapeutic phase. (a) Intrabony three-wall defects subjected to regenerative periodontal therapy at maxillary right second premolar and first molar. (b) Wound closure upon application of enamel matrix derivatives onto the root surfaces. (c and d) Minimally invasive access to the three-wall intrabony defect at the mandibular right first molar. (e) Wound closure upon application of enamel matrix derivatives onto the root surface.




Figs 2-8a to d Periodontal parameters in the course of periodontal therapy. (a) Periodontal inflamed surface area (PISA)113 projected to the palm of a hand; reduction of PISA during therapy up to 24 months after surgical treatment (supportive periodontal therapy [SPT]). (b) Attachment loss, (c) probing pocket depth, and (d) bleeding on probing (BOP) during active periodontal therapy and SPT (graphic illustration by Parostatus.de).






Figs 2-9a to f Radiographic images of the therapeutic outcome 2 years after regenerative surgical therapy. (a) Baseline radiograph: maxillary right second premolar to third molar. (b) Radiograph 2 years after surgery: maxillary right second premolar to second molar, with radiographic bone fill indicating periodontal regeneration upon application of enamel matrix derivatives. (c) Baseline radiograph: maxillary right second premolar to second molar. (d) Radiograph 2 years after surgery: maxillary right second premolar to second molar. (e) Baseline radiograph: mandibular right second premolar to second molar. (f) Radiograph 2 years after surgery: mandibular right second premolar to second molar, with radiographic bone fill indicating periodontal regeneration upon application of enamel matrix derivatives.






Figs 2-10a to f Case of a male patient (see Fig 2-3): examinations 10 months after surgery. (a) First and fourth quadrant. (b) Second and third quadrant. (c) Frontal view. (d) Maxillary arch. (e) Mandibular arch. (f) Measurements of the clinical attachment level, including tooth migration and mobility, furcation involvement, and bleeding on probing (graphic illustration by Parostatus.de).

Fig 2-11 Periodontal risk assessment.114 After active periodontal therapy (nonsurgical and surgical therapy), a moderate individual risk was evaluated. The patient attends the supportive periodontal therapy program four times a year (graphic illustration by Parostatus.de). (BOP, bleeding on probing; PD, probing depth.)
Diagnosis and classification system
Clinical and radiographic examinations are necessary to determine the periodontal diagnosis, and both are prerequisites for any therapeutic consideration. Diagnoses of periodontal diseases are described in the recently published classification system, which includes staging and grading of periodontitis. This classification system considers the severity and complexity of periodontal destruction by graduating into four stages (I, II, III, and IV) as well as the disease progression graduated into three grades (A, B, and C).115,116 Furthermore, extended and distributional patterns of periodontitis are also characterized.116 In the context of staging, periodontitis stage I is considered as initial periodontitis, stage II as moderate periodontitis, stage III as severe periodontitis with potential for tooth loss, and stage IV as advanced periodontitis with extensive tooth loss and potential for loss of dentition. The grading system refers to risk factors or actual evidence of progression of periodontitis and is divided into slow, moderate, or rapid rates of progression. Recognized risk factors, such as smoking or metabolic control of diabetes, affect the progression of periodontitis and may contribute to the conversion to the next higher grade.117 Besides the description and characterization of the plaque-induced inflammatory disease periodontitis, the classification system also comprises other periodontal diseases and conditions such as gingival diseases (dental plaque-induced and nondental plaque-induced gingivitis), necrotizing periodontal diseases, periodontitis as a manifestation of systemic disease, and recessions (including noncaries cervical lesions, periodontal-endodontic lesions, periodontal abscesses, and peri-implant diseases and conditions).118–120 More detailed information on periodontitis stages and grades is shown in Tables 2-1 and 2-2, respectively.
Table 2-1 Overview regarding the periodontitis stages according to the Classification of Periodontal and Peri-implant Diseases and Conditions (2018)115,116
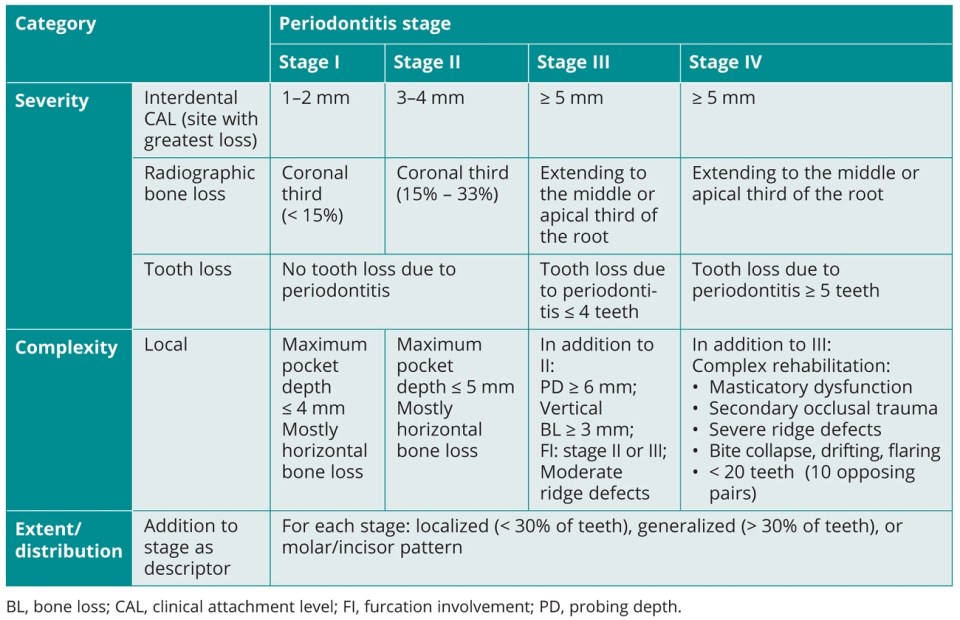
Table 2-2 Overview regarding the periodontitis grades according to the Classification of Periodontal and Peri-implant Diseases and Conditions (2018)115,116

Prevention and therapy of periodontal diseases
Periodontal diseases and prevention
Gingivitis and periodontitis are understood as a continuum of a chronic inflammatory disease entity. In the development of periodontitis, the chronic inflammatory process in response to the dental plaque biofilm leads to the development of a dysbiosis within the biofilm along with irreversible periodontal tissue destruction.10,121
In the context of periodontal diseases, there are two different preventive strategies: primary and secondary prevention. Primary prevention of gingivitis aims to maintain gingival health, and therewith, protect the gingival tissues from developing clinical signs of inflammation by executing sufficient individual oral hygiene procedures including regular professional mechanical plaque removal.121 Gingival health is associated with an inflammatory infiltrate as host response consistent with homeostasis in the presence of a symbiotic biofilm, and the definition describes a condition with no attachment loss, shallow periodontal pockets (≤ 3 mm), less than 10% bleeding on probing, no erythema or edema, no patient symptoms, and no radiographic bone loss in a patient with an intact periodontium.12,122 This means that gingival health is a condition consistent with a low grade of physiologic inflammatory response, which includes, amongst others, a neutrophil infiltrate and the expression of antimicrobial peptides by not only neutrophils but also periodontal soft tissue cells.11,123,124 In addition, gingival health may also be present in a patient with a reduced periodontium (in the presence of recessions or in the case of a stable periodontitis patient).122 In primary prevention of gingivitis, the goal is to maintain this state of gingival health in individuals with an intact or a reduced periodontium.
Prevention of periodontitis may be subclassified as primary and secondary preventive approaches. For the primary prevention of periodontitis, the obviation of periodontitis may be accomplished by preventing the continuation of the inflammatory process, eventually leading to the destruction of periodontal attachment. This means the treatment of gingivitis aiming to avoid the potential conversion from gingivitis to periodontitis in a susceptible patient.121 Currently, the exact molecular mechanisms of when and how a case of gingivitis converts to a case of periodontitis are not fully understood. Therefore, treatment of patients with gingivitis, including disruption and/or removal of the dental plaque biofilm, is the main aspect in the primary prevention of periodontitis.
Secondary prevention of periodontitis, in contrast, refers to the prevention of disease recurrence.125 More precisely, the aim of secondary prevention is to avoid the occurrence of gingival inflammation leading to additional periodontal attachment loss in a periodontitis patient who has been successfully treated.121
For the prevention of periodontal diseases, it is important to understand that, in addition to the dental plaque biofilm, there are further risk factors, such as smoking and diabetes mellitus, influencing the susceptibility to gingivitis and periodontitis. To establish a robust preventive strategy, it is mandatory to be aware of the variety of etiologic factors that may affect the periodontal tissues and eventually lead to disease.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


