Armamentarium
|
History of the Procedure
The primary goal of temporomandibular joint total joint replacement (TMJ TJR) is the long-term restoration of mandibular function and form. The fundamental tasks of mastication, speech, deglutition, and airway support are maintained by TMJ function and form. Over a lifetime, this places the TMJ complex under more cyclical loading and unloading than any other joint. Therefore, to provide successful function and form long-term outcomes, any TMJ TJR device must be capable of dealing with the anatomic, functional, and aesthetic discrepancies that indicated its use. Outcome data confirm that any pain relief attained must be considered of only secondary benefit. Despite persistent but reduced chronic pain, long-term increased mandibular function and form improvement have been reported, resulting in quality-of-life improvement for 85% of patient-fitted TMJ TJR patients studied. Based on the literature to date and the accepted orthopedic criteria for the development and utilization of successful TJR devices, this chapter discusses the use of a patient-fitted (custom) system as the TMJ TJR option of choice for successful long-term management of end-stage TMJ disease.
History of the Procedure
The primary goal of temporomandibular joint total joint replacement (TMJ TJR) is the long-term restoration of mandibular function and form. The fundamental tasks of mastication, speech, deglutition, and airway support are maintained by TMJ function and form. Over a lifetime, this places the TMJ complex under more cyclical loading and unloading than any other joint. Therefore, to provide successful function and form long-term outcomes, any TMJ TJR device must be capable of dealing with the anatomic, functional, and aesthetic discrepancies that indicated its use. Outcome data confirm that any pain relief attained must be considered of only secondary benefit. Despite persistent but reduced chronic pain, long-term increased mandibular function and form improvement have been reported, resulting in quality-of-life improvement for 85% of patient-fitted TMJ TJR patients studied. Based on the literature to date and the accepted orthopedic criteria for the development and utilization of successful TJR devices, this chapter discusses the use of a patient-fitted (custom) system as the TMJ TJR option of choice for successful long-term management of end-stage TMJ disease.
Indications for the Use of the Procedure
Salvage management of the following end-stage pathologic conditions are considered indications for patient-fitted TMJ TJR.
Inflammatory Arthritis Involving the TMJ Not Responsive to Other Modalities of Treatment
Inflammatory arthritis involves a local, synovial tissue–mediated, destructive systemic disease process; therefore, orthopedists opt for TJR because the long-term results are predictable. When the mandibular condyle is extensively damaged, degenerated, or absent as the result of inflammatory arthritic conditions, TMJ TJR will provide a similar predictable approach to achieve optimal functional and symptomatic improvement in light of the disease process.
Recurrent Fibrosis or Bony Ankylosis Not Responsive to Other Modalities of Treatment
The traditional management of TMJ bony ankylosis has been gap arthroplasty with autogenous tissue grafting or alloplastic hemiarthroplasty. Despite an autogenous grafting technique, the ability to create form, fixation, and stabilization of any autogenous tissue at surgery necessarily delays early mandibular functional rehabilitation. The consequences of autogenous bone graft mobility during the initial stages of healing severely compromise its incorporation into the host bone by impeding or hindering graft neoangiogenesis. Early mandibular mobility will result in graft/host interface failure.
In a patient with TMJ bony ankylosis or reankylosis, placing an autogenous bone graft into an area where reactive or heterotopic bone is forming intuitively makes no sense. Therefore, in light of biologic considerations, TMJ TJR should be considered in the management of TMJ ankylosis and reankylosis cases.
Failed Tissue Grafts (Bone and Soft Tissue)
Successful outcome with autogenous tissue grafting requires that the recipient site provide a rich vascular bed. Unfortunately, the scar or pathology-damaged tissue encountered in the multiply operated patient and many end-stage disease TMJ cases does not provide an environment conducive to the predictable success of free or the occasional vascularized autogenous tissue graft. Marx reported that capillaries can penetrate a maximum tissue thickness of 180 to 220 µm, whereas the thickness of scar tissue surrounding previously operated bone averages 440 µm. This may account for the clinical observation that free autogenous tissue grafts, such as cartilage, costochondral, and sternoclavicular grafts, often fail in multiply operated patients or those with extreme anatomic bony architectural discrepancies resulting from end-stage pathology. Further, anatomic architectural variances found in such cases make “off the shelf” or stock TMJ TJR devices difficult to adapt, fixate, and stabilize for function. Therefore, patient-fitted (custom) TMJ TJR appears to provide the soundest option in this situation.
Failed Alloplastic Joint Reconstruction
Due to the osteolysis that occurs around a failed alloplast resulting in substantial host bone anatomic architectural discrepancies, it is difficult to adapt and stably fixate autogenous tissues or stock TMJ TJR devices to these host bone remnants. Further, the foreign body giant cell reactions associated with failed or failing devices provide an unfavorable environment for the introduction of an autogenous graft. Therefore, it appears that patient-fitted (custom) TMJ TJR devices provide more predictable outcomes than do autogenous tissues in such cases.
Loss of Vertical Mandibular Height or Occlusal Relationship Due to Bony Resorption, Trauma, Developmental Abnormalities, or Pathologic Lesions
Loss of posterior mandibular vertical dimension due to the developmental abnormalities, end-stage TMJ pathology, neoplasia, or traumatic injury results in a discrepancy in mandibular function and form. In cases where the diagnosis couples the etiology directly with the TMJ components, TMJ TJR rather than osteotomy or autogenous tissue reconstruction should be considered.
Limitations and Contraindications
Age of the Patient
Because TMJ TJR devices have no intrinsic growth potential, the benefits of their utilization in growing patients over autogenous tissue must be considered carefully. However, literature suggests that further investigation into the use of TMJ TJR in growing patients is warranted.
Mental Status of the Patient
Is the patient psychologically prepared to cope with the permanent loss of a body part and understand that revision or replacement surgery may be required in the future? Does the patient have unrealistic expectations of complete relief of pain and normal jaw function after alloplastic TMJ replacement? Is the patient willing or able to do the postimplantation physical therapy required to obtain maximum functional benefit from the procedure?
Many multiply operated, functionless TMJ patients require prereplacement psychological counseling in order for them to accept the limitations of further surgery.
Uncontrolled Systemic Disease
As with any form of an alloplastic implant (dental, orthopedic, TMJ) in patients with potentially compromising medical conditions, such disorders must be brought under control and the risk/benefit ratio determined for the individual patient before implantation can proceed. These patients, if implanted, should be well informed preoperatively and monitored closely postoperatively for potential complications.
Active Infection at the Implantation Site
As with any alloplast material, introduction into an infected or contaminated area can result in failure of the device to stabilize, leading to micromotion and its ultimate malfunction under functional loading.
Documented Allergy to the Device Materials
Documented allergy to titanium, titanium alloy, cobalt-chrome-molybdenum alloy, and ultra-high molecular weight polyethylene is rare. Although 12% to 15% of the population can be sensitive to the nickel alloy in cobalt-chrome-molybdenum components, far fewer reports of such allergic reactions have been reported in the orthopedic literature. Patients with documented allergy to the component materials of any device should not be exposed to that material in any new device.
Technique: Custom TMJ Total Joint Replacement
Step 1:
Patient Preparation
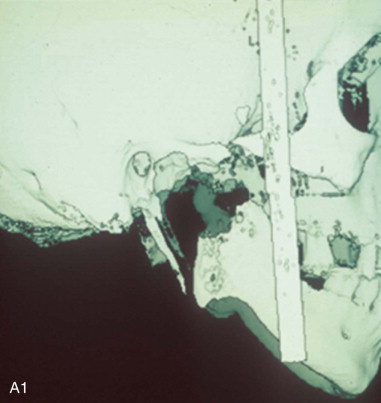
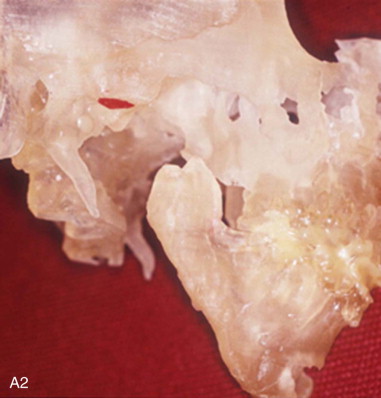
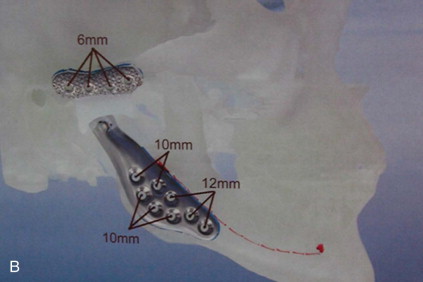
A diagnosis indicating the necessity for TMJ TJR is made, the patient is fully informed, the patient’s consent to proceed is made, and then the process of designing and manufacturing a custom TMJ TJR device begins. The patient undergoes a protocol computed tomography (CT) scan ( Figure 130-1, A1 ) from which a stereolithic acrylic (SLA) model is developed ( Figure 130-1, A2 ). The surgery will be planned and the TMJ TJR fossa and ramus components are designed and manufactured from this model. The surgeon uses the SLA model for planning and applicable modification in conjunction with a design engineer. The surgeon then approves the implant design ( Figure 130-1, B ), and the components of the custom TMJ TJR are manufactured and sterilized. The sterile components, design schematic including exact fixation screw lengths ( Figure 130-1, B , presented later), and implantation kit are sent to the surgeon’s hospital. The surgeon implants the components ( Figures 130-3 and 130-4 , presented later) and begins follow-up procedures.
Patients should be advised to wash their hair with mild shampoo before surgery and not use any hair sprays, gels, or facial makeup. Prophylaxis with a broad-spectrum antibiotic should be started 1 hour before incision. Hair in the area of the preauricular incision should be sheared, not shaved. Any remaining hair, eyes, auditory canal(s), and mouth should be appropriately isolated from the surgical field. All instruments used intraorally or to harvest autogenous abdominal fat must be strictly isolated from those used to implant the TMJ TJR device components.
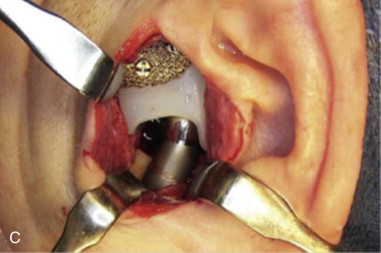
The device components should be immersed in a 1-g vancomycin/250 cc saline solution during the procedure; then that solution is used to irrigate the preauricular and retromandibular incisions before closure ( Figure 130-1, C ).
Step 2:
Incisions and Dissection
Find the crease between the helix and the preauricular skin, and mark a line from the top of the helix to the lobe. In previously operated patients, use the scar to make this incision. In patients with multiple scars, excise the scarred tissue with the initial incision and revise the scar at closure. The superior aspect of the incision should be extended anteriorly and superiorly 4 cm at a 45-degree angle to the zygomatic process of the temporal bone. Inject a vasoconstrictor (e.g., 1 : 200,000 epinephrine solution) along the line to be incised to decrease bleeding. Wait for its effect (3 minutes). Apply traction to each end of the incision line with single-ended skin hooks. With a #15 blade, incise the skin and subcutaneous tissue along the incision line. At the superior aspect of the incision, spread the tissue with a curved mosquito hemostat to find the superficial layer of the temporalis fascia. This is the obvious tough, shiny, white, sinewy-appearing dense tissue. Once this layer has been found, slide the hemostat inferiorly along the top of this fascia to the area of the zygomatic arch.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


