The metabolism of calcium and phosphorus
Publisher Summary
This chapter discusses the metabolism of calcium and phosphorous. Calcium and phosphorus are among the most abundant elements in the body, and they constitute the greater part of the mineral phase of the hard tissues. A 70 kg man contains some 1150 g of calcium and about 700 g of phosphorus, representing about 1–7 and 1–0% of the total body weight. Some 600 g of phosphorus are located in the skeletal and dental tissues as inorganic (ortho)-phosphate, while the remaining 100 g are present in various forms in the soft tissues and extra-cellular fluids. Apart from the inorganic phosphates, which make an appreciable contribution to the buffering capacity of the extracellular fluids, phosphorus-containing derivatives of carbohydrates, lipids, and proteins all have important functions in the body. Phosphoric acid is an essential constituent of nucleotides, including the high-energy di- and triphosphates and the nucleic acids. Ionic calcium is essential for muscular contraction, transmission of nerve impulses, neuromuscular irritability, and for maintaining the integrity of cell membranes; it is also necessary for the clotting of blood and milk. Calcium is almost entirely extracellular in its distribution in contrast to phosphorus, which is more abundant than calcium in soft tissues and is largely intracellular.
Calcium and phosphorus metabolism
Vitamin D and calcium metabolism
Vitamin D has long been recognized as an essential factor in the absorption of calcium from the gut. As mentioned in Chapter 12, deficiency of this vitamin in infants and children causes rickets, which is characterized by abnormal endochondral calcification, resulting in bones that are hypocalcified and soft. Rickets can be induced experimentally in rats fed on diets lacking vitamin D or low in phosphorus.
Transport of calcium across the gut wall completely stops in animals that have been depleted of vitamin D but begins again, after a definite time-lag, when vitamin D is restored to their diet. However, if vitamin D is added in vitro to the everted gut sac from an animal starved of the vitamin, it has no effect in stimulating transport of calcium against a concentration gradient. This apparent paradox was resolved by a series of investigations, the success of which depended upon, firstly, the ability to produce labelled cholecalciferol with a sufficiently high specific activity for it to be studied at physiological dose levels and, secondly, the development of chromatographic techniques for the separation and subsequent identification of nanogram (10−9 g) levels of steroids and related compounds. By means of these techniques it has been shown that cholecalciferol is first converted in the liver to 25-hydroxycholecalciferol (25-HCC) (Figure 30.1), which is more biologically active than cholecalciferol itself. This substance is then hydroxylated further, in the kidney, to produce 1,25-dihydroxycholecalciferol (1,25-DHCC), the most potent antirachitic substance known. Furthermore, 1,25-DHCC, unlike cholecalciferol itself, can stimulate calcium transport in isolated gut sacs in vitro. The 1,25-DHCC finds its way to the nucleus of the surface intestinal cells, where it unmasks a specific gene which, by transcribing the appropriate mRNA, codes for a calcium-binding protein CaBP. This protein is located in the intestinal brush border and effects transport of calcium across the wall of the intestine. The need for two successive hydroxylations of cholecalciferol in the liver and kidney and the subsequent migration of the 1,25-DHCC to the target cells, followed successively by unmasking, transcription and protein synthesis, accounts for the time-lag observed before cholecalciferol produces its effect in deficient animals. Administration of actinomycin D, an inhibitor of protein synthesis, has been shown to block the physiological response to vitamin D by preventing the synthesis of the calcium-binding protein.
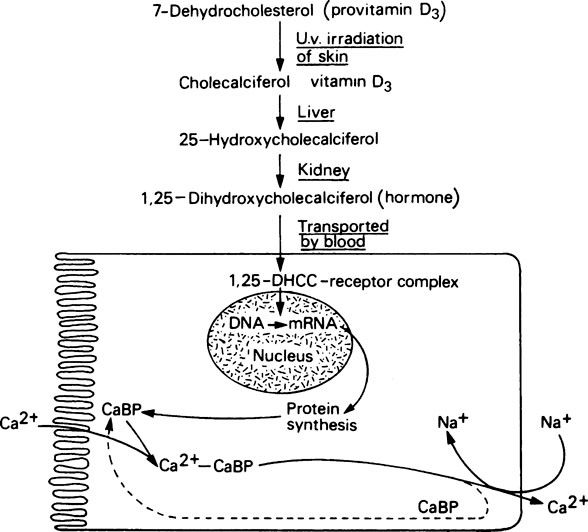
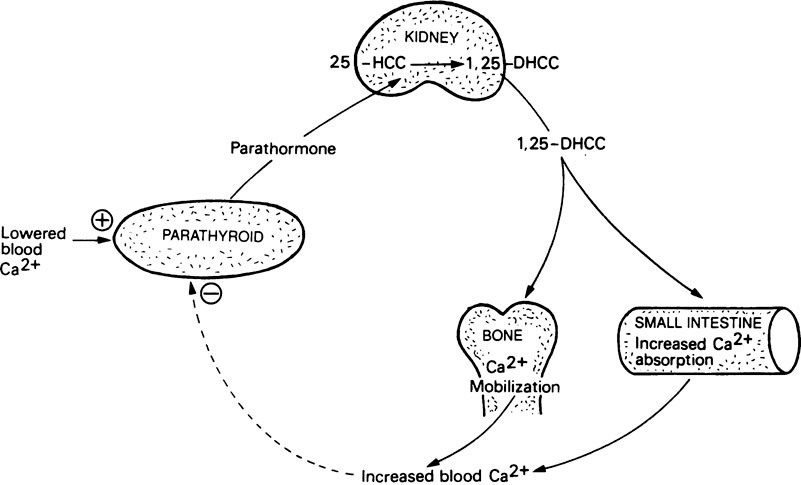
Dihydroxycholecalciferol is able to act on a number of tissues with columnar epithelial cells, including intestinal mucosa, kidney tubules, the shell gland of birds and probably also various types of bone cell where it may assist the synthesis of osteocalcin (page 161). Its mode of action is very similar to that of steroid hormones (Figure 30.1). In this respect its precursor, vitamin D3, may be considered to function as a prohormone rather than a vitamin. The ability of 1,25-DHCC and other metabolites of vitamin D3 to act on bone and kidney cells, as well as those of the intestine, means that vitamin D plays a key role in calcium and phosphorus metabolism (Figure 30.2).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


