The formation and properties of dental plaque
Publisher Summary
This chapter discusses the formation and the properties of dental plaque. When a tooth is brushed with an abrasive agent, it is possible to remove all the organic material that has accumulated on the surface and expose the naked apatite crystals. If the tooth is then allowed to interact with saliva, either naturally in the mouth or in a bacteria-free artificial system, a thin acellular surface film forms within a few minutes. This material is known as the acquired pellicle. This integument is about 10μm thick and consists of a surface and subsurface layer. Initially, the surface is gelatinous, but it hardens with time and may become calcified. Pellicle stains positively for carbohydrate and protein, and appears to be derived from the salivary proteins. Its amino-acid composition is similar to that of a typical glycoprotein, and it contains hexoses, hexosamines, and fucose. However, the glucose/galactose ratio differs from that in saliva. The pellicle consists of a specific salivary glycoprotein fraction whose oligosaccharide side chains are resistant to enzymic degradation. Calcium and phosphate ions are involved in the precipitation of salivary proteins to form the pellicle and the protein matrix of plaque. Calcium and phosphate each form ionic bonds with oppositely charged groups in the salivary proteins, and this may lead to the coprecipitation of protein with insoluble calcium phosphate salts. Plaque contains high concentrations of calcium and phosphate as compared with saliva, and salivary glycoproteins have been found to be rapidly and selectively bound by hydroxyapatite powder and powdered enamel, the adsorbed material having a similar electrophoretic mobility to protein collected from extracted teeth.
The mechanism of pellicle formation is far from clear but there is no doubt that it forms rapidly on all enamel surfaces. Although there is justification for regarding it to be normal for a tooth to be covered with pellicle, there is considerable controversy as to whether this integument has any function. It may protect the teeth against acid attack, and abraded areas of enamel are undoubtedly more susceptible to decalcification, but it has not been proved whether this is due to removal of the pellicle or of some other components of the enamel surface. The presence of the protein–calcium phosphate complex increases the resistance of enamel to dissolution and, since enamel defects are often filled with insoluble protein, pellicle formation may assist in subsequent recalcification (page 513). Another suggestion is that, because it provides a continuous layer of protein at the enamel–gingival interface, it may be implicated in the adhesion of the gingival epithelium to the tooth.
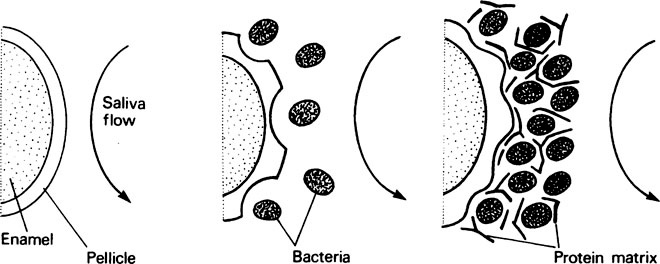
Newly formed pellicle is quickly invaded by bacteria (Figure 34.1) derived from the saliva, the adjacent soft tissues and any defects in the enamel surface, and their growth produces discrete colonies which eventually fuse to produce a bacterial mass. At first the bacteria rest on top of the pellicle but soon come to lie in saucer-shaped depressions, which suggests that the pellicle is being actively metabolized. During colonization a matrix of protein is deposited around the bacteria and, in time, the bacterial-protein complex becomes detectable as dental plaque. This is particularly noticeable around the gingival margins and can be demonstrated by the use of suitable disclosing solutions, such as erythrosine or basic fuchsin. Crystals of calcium phosphate may form within mature plaque but take weeks to appear; plaque which is obviously calcified is called calculus (page 514).
The plaque flora – cell adhesion
1. The initial reversible deposition (or capture) of a single cell on to the surface. This depends on non-covalent physicochemical forces, particularly ionic interactions and Van der Waals’ forces (page 15).
2. The time-dependent irreversible adhesion of the captured cell. Surface stabilization depends on calcium bridging, hydrophobic interactions and the formation of covalent bonds with surface groups at distances of less than 0·4 nm, or the establishment of polymer bridging at distances up to 10 nm.
3. The colonization of the surface. This involves the growth and multiplication of cells and the development of cell-to-cell contacts which are characterized by the appearance of bacterial extracellular products, mainly proteins and polysaccharides.
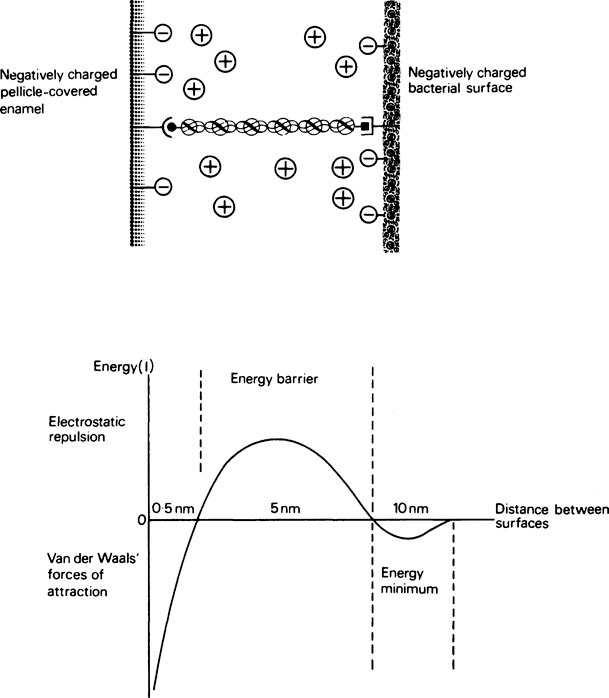
Hydroxyapatite at pH 6·5 carries a net positive charge and readily attracts bacteria, most of which have an overall negative charge on their surface owing to their outer covering of teichoic acid, a polymer of glycerol or ribitol units joined by phosphodiester bonds. The binding of salivary proteins to the hydroxyapatite converts the positive surface to a negative one and treatment with saliva makes the bacteria slightly less negative, both surfaces now having a degree of hydrophobic character. Deposition of bacteria under these conditions will depend on a balance between negative charge repulsion, Van der Waals forces of attraction and hydrophobic interactions between specific groups on the bacterial and salivary-coated hydroxyapatite surfaces. Successful ‘capture’ holds the cell in a minimum-energy position about 10 nm from the surface (Figure 34.2) on which it may become stabilized by bridging the gap with polymeric material, usually protein, or by bacterial ‘appendages’ which include flagellae, pili and fimbriae. Adhesion may be increased by the presence of aggregating molecules in the saliva. However, saliva-induced aggregation may also serve to prevent surface attachment by forming bacterial clumps which are more readily removed by salivary flow, in much the same way that immunoglobulins help to immobilize invading bacteria (page 381). It may be concluded that saliva reduces overall deposition and encourages the attachment of organisms which are part of the ‘normal’ plaque flora rather than ‘opportunistic’ pathogens such as S. mutans. The enamel surface, once it is colonized, unlike other epithelial surfaces such as the tongue and oral mucosa, is unable to shed its bacterial load by surface desquamation. Consequently, the rate of initial deposition is roughly proportional to the rate of colonization and the bacteria remain in place more or less permanently unless they are removed by the forces of mastication or vigorous toothbrushing. Plaque films several millimetres in thickness can develop on inaccessible surfaces and in stagnation sites.
 –, specific recognition sites;
–, specific recognition sites;  polymer bridge; –
polymer bridge; – , fixed negative charge on surface;
, fixed negative charge on surface;  , adsorbed counter ions in medium
, adsorbed counter ions in mediumStay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses