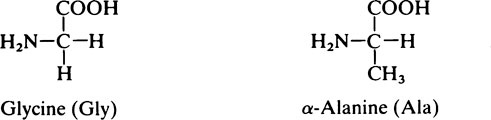The amino acids
Publisher Summary
This chapter discusses amino acids, which are the building materials for the proteins that are the most characteristic constituents of living matter and among the most complex substances known. Proteins are irregular polymeric compounds made up from a selection of 20 different amino acids joined together by peptide linkages. They may contain anything from one hundred to several thousand amino acid units joined in a specific genetically determined order, and each protein has unique chemical and biological properties. The amino acids are amphoteric compounds and contain both a potentially basic amino (—NH2) group and a potentially acidic carboxyl (—COOH) group. Certain features of the individual amino acids have an important bearing on the structure and function of proteins. The properties and reactions of the amino acids may be broadly divided into three types: those due to the presence of (1) the acidic α—COOH group, (2) the basic α-NH2 group, and (3) the side-chain grouping.
< ?xml:namespace prefix = "mml" />
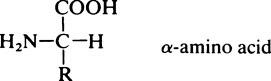
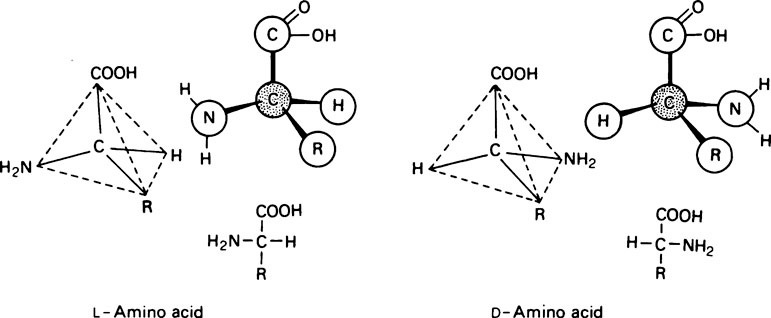
All the amino acids except glycine (in which R = H) contain at least one asymmetric carbon atom, i.e. one that has four different groups attached to it and exist in stereoisomeric forms. The amino acids in proteins belong to the L-series, since they are related in their three-dimensional configuration to the reference compound L-glyceraldehyde (page 91). The D and L forms, which are mirror images of each other and cannot be superimposed, may be represented on a flat sheet of paper as shown in Figure 4.1. The hatched circle represents the asymmetric C atom which is in the plane of the paper, and the –COOH and R– groups of the amino acid project behind the plane of the paper and the –H and –NH2 in front of it.

The amino acids may be divided into:
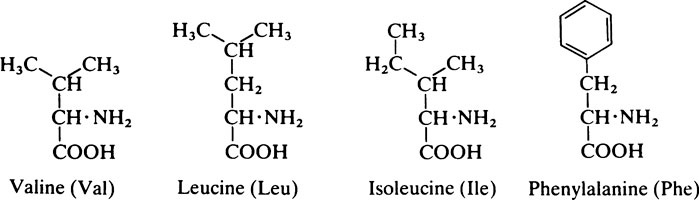
1. Those with a non-polar hydrocarbon side chain: glycine, alanine, valine, leucine, isoleucine, phenylalanine and proline.
2. Acidic amino acids which have two acidic groups and only one basic group: aspartic acid and glutamic acid.
3. Basic amino acids which possess two basic and only one acidic group: lysine, arginine and histidine.
4. Amino acids with un-ionized but polar substituents in their side chains, e.g.:
| hydroxyl (–OH) | serine, threonine and tyrosine |
| sulphydryl (-SH) | cysteine |
| acid amide (-CONH2) | asparagine and glutamine |
| others | tryptophan and methionine (contains S) |
Characteristics of the individual acids
Acidic amino acids
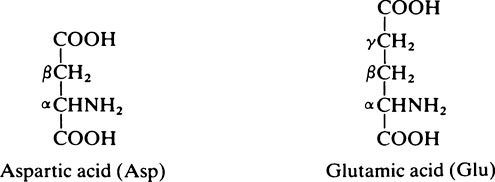
and the α–COOH group are unreactive since they are combined in peptide linkage (page 39) with the adjacent amino acids. However, the second (β or γ) –COOH group remains free and reactive.
A carboxylated derivative of glutamic acid, γ-carboxyglutamic acid, has been found in a number of proteins, notably in prothrombin and other blood-clotting factors (page 391).
Basic amino acids

chains. Its derivatives include hydroxylysine, desmosine and isodesmosine (Chapter 27). Ornithine, a lower homologue of lysine, which contains five instead of six carbon atoms is not found in proteins although small quantities occur free in the liver where it is involved in the synthesis of urea.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses