Introduction
Midpalatal suture expansion could induce osteogenesis to correct maxillary insufficiency; cartilage formation could also be induced, and lower-magnitude forces might generate a preferable response pattern. In this study, we aimed for an enhanced understanding of the cartilage formatting effects of expansion.
Methods
Thirty 6-week-old male C57BL/6 mice were randomly and evenly assigned to 3 groups; the animals in each group received a sustained suture expansion at 0, 10, and 20 g, respectively. Ten additional mice were fed the same as the baseline controls and received no expansion. After 7 days, the animals were killed; coronal paraffin sections were stained using toluidine blue and safranin-O. The proliferating cell nucleus antigen, the nuclear antigen Ki-67, alkaline phosphatase, and matrix metalloproteinase 13 expressions were visualized with immunohistochemistry. All data were analyzed statistically, and the differences were considered significant at P <0.05.
Results
Compared with the control, the cartilage matrix volume was significantly increased by the 20-g expansion, showing increased cartilage matrix and hypertrophic chondrocytes with the highest matrix metalloproteinase 13 expression. The 10-g expansion formed condensed proliferating chondrocyte masses, within which the highest percentages of proliferating cell nucleus antigen and Ki-67 positive cells were present. The 10-g and the 20-g expansions equally intensified the alkaline phosphatase expression.
Conclusions
The lower expansion (10 g) promoted chondrocyte proliferation and induced a more preferable suture cartilage response pattern compared with the higher expansion (20 g), which just increased the cartilage matrix production.
Highlights
- •
Suture expansion could correct maxilla deficiency.
- •
Lower (10 g) and higher (20 g) expansions could induce cartilage formation in the midpalatal suture.
- •
Suture cartilage-remodeling patterns vary with different expansive force magnitudes.
- •
Lower expansion force increased the suture chondrocyte proliferation.
- •
Higher expansion force promoted the secreting function of the chondrocytes and increased the cartilage matrix.
Midpalatal suture expansion has been well documented as a clinical approach for correcting maxillary volume insufficiency because of its bone formation ability. The expansion protocols differ from each other by the use of strong forces for a quick expansion or lower forces for a stable expansion, both of which induce considerable immediate osteogenesis and achieve the treatment goals. The latter protocol has been applied frequently in orthodontics, demonstrating less relapse after the mechanical expansion devices were removed. The stable lower-force expansion produces approximate osteogenesis because it stimulates the secondary cartilage’s osteogenic differentiation of the secondary cartilage. An early study showed that a suture expansion of 10 to 20 g in 4-week-old male Wistar rats increased osteogenesis between the cartilage and the precartilage, and a 20-g expansion induced bone formation between the cartilage and the bone. A 50-g expansion also induced bone formation within the cartilage at an early stage, whereas a 56-g expansion in 6-week-old male C57BL/6 mice induced bone formation in the periosteal vicinity.
The endochondral osteogenesis in the secondary suture cartilage was for a long time documented as a source of the expansion-induced bone formation ; however, the response of the suture cartilage, per se, at different expansions is unclear. The 10- to 20-g expansive forces decreased the cartilage matrix amount and the type II collagen expression by directing the osteochondral progenitor cells to an osteogenic commitment, and the 50-g expansive force partially replaced the cartilage with highly proliferative preosteoblasts. The 56-g expansion in mice completely removed the cartilage; however, as the force dissipated, the cartilage reemerged on the oral side of the suture. The 20-g suture expansion in 6-week-old Wistar rats for 5 days induced chondrocyte proliferation with an increased proliferating cell nucleus antigen (PCNA) positive cell count. The preliminary data from the previous pilot studies showed that suture expansion induced cartilage formation in 6-week-old male C57BL/6 mice. Cartilage formation could be induced under suture expansion, and lower-magnitude forces might generate a preferable response pattern.
The PCNA is an index of the Gap1 (G1) and the synthesis (S) phases of the cell cycle ; its expression level was intensified in highly proliferating chondrocyte progenitor cells. The nuclear antigen Ki-67 expression increased only in cells that were in the mitosis period, which specifically distinguished the proliferating cells. When the chondrocytes matured functionally, the hypertrophic cells showed active secretion of the cartilage matrix. Glycosaminoglycan was produced to form the major composition of the cartilage, which could be easily distinguished by its metachromatic property when treated with toluidine blue or safranin-O. Type II collagen was laid down to form the unique component of the cartilage, newly secreted collagen fibrils, which were cleaved, reassembled, and remodeled with glycosaminoglycan to form a new cartilage matrix, with help from matrix metalloproteinase 13 (MMP-13).
To better understand how a lower and stable expansion affected the response of suture cartilage, and whether it resulted in a more preferable suture cartilage response, constant 10-g and 20-g continuous expansive forces were delivered to the midpalatal suture of the mice for a week using custom manufactured nickel-titanium helix springs (Baoji Hexin Titanium Products, Baoji, Shanxi, China). Toluidine blue and safranin-O staining were used to distinguish the cartilage. The cartilage volume, the chondrocyte count, and the chondrocyte morphology were analyzed. The fluorescent in situ hybridization against the type II collagen mRNA was used to confirm the chondrocyte secretion. The PCNA and Ki-67 expressions were visualized by immunohistochemistry, and the percentages of the proliferating chondrocytes were calculated. The osteogenic differentiation in the suture region was evaluated by visualizing the alkaline phosphatase (ALP).
Material and methods
Thirty C57BL/6 male mice (6 weeks old; weight, 23-25 g) were randomly and evenly divided into sham control, 10-g expansion, and 20-g expansion groups. Under anesthesia, each animal received a custom manufactured nickel-titanium wire spring, which was precalibrated by the manufacturer to deliver a stable force of 0, 10, or 20 g, respectively, in the deformation range of 0.5 to 2.0 mm ( Fig 1 ). The springs were bonded bilaterally to the first and second maxillary molars with light-cured composite resin (Transbond XT Light Cure Adhesive Paste; 3M Unitek, Monrovia, Calif). The springs in the 10-g and 20-g groups had been activated during the bonding. The springs in the sham control group were passively bonded with no force. An additional 10 mice, which received no spring, were raised for the baseline control.
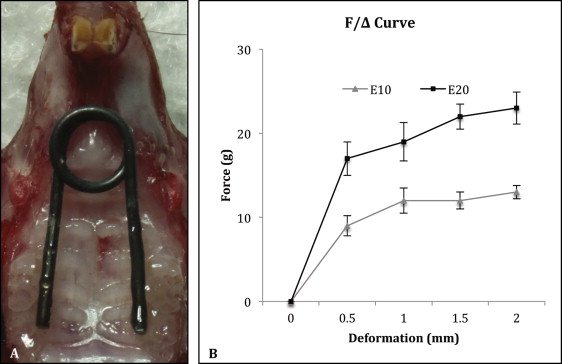
All animals were fed an identical ground diet, and their behavior and body weight were monitored daily. Seven days after bonding the springs, they were killed. The maxillae were dissected, fixed, and embedded in paraffin. Serial coronal sections were made in the coronal plane at the maxillary first molar region as previously described. All procedures and protocols were approved by the institutional research board of State Key Laboratory of Oral Diseases, Sichuan University, in China.
The suture cartilage was distinguished using toluidine blue (Sigma-Aldrich, St Louis, Mo) staining. Blending a 5-mL toluidine blue stock solution (1 g of toluidine blue in 100 mL of 70% ethanol) with a 45-mL sodium chloride (Sigma-Aldrich) solution (1% sodium chloride in distilled water) created the staining working solution (pH 2.3). The coronal sections at the first molar region were deparaffinized and rehydrated, treated with the staining working solution for 2 minutes at room temperature, rinsed with distilled water, destained with 75% ethanol for approximately 10 seconds, dehydrated with absolute ethyl alcohol for 3 minutes, treated twice with xylene for 5 minutes, and sealed with a commercial xylene-based resin sealant (Eukitt; Sigma-Aldrich).
Metachromasia with safranin-O (Sigma-Aldrich) was used to visualize the secreted cartilage matrix. The deparaffinized and rehydrated sections were treated with Weigert’s iron hematoxylin (Sigma-Aldrich) for 30 seconds, destained with an acid ethanol solution (1 mL of concentrated hydrochloric acid in 399 mL of 70% ethanol), and differentiated with 1% ammonia (Sigma-Aldrich) water to brighten the nuclei. The sections were treated with 0.1% fast green (Sigma-Aldrich) solution for 8 minutes, destained with 1% acetic acid (Sigma-Aldrich) solution, then treated with 0.1% safranin-O solution, rinsed, destained with 90% ethanol, dehydrated, cleared, and sealed.
The PCNA, Ki-67, ALP, and MMP-13 expressions were visualized using immunohistochemistry, as previously described. The serial sections were boiled for 3 minutes in 10 m mol/L of citrate buffer (pH 6.0) to retrieve the antigens. Then, the sections were blocked with 3% normal goat serum (MP Biomedicals, Auckland, New Zealand) for 30 minutes at room temperature, incubated overnight at 4°C with rabbit anti-mouse PCNA (07-2162; Millipore, Temecula, Calif) (1:200), rabbit anti-mouse Ki-67 (AB9260, Millipore) (1:200), rabbit anti-mouse MMP-13 (ab39012; Abcam, Cambridge, United Kingdom) (1:200), or rabbit anti-mouse ALPL (TA307702; OriGene Technologies, Rockville, Md) (1:400) antibodies. The primary antibody reactions were visualized with an avidin-biotin immunoperoxidase secondary detection system (DAB150 kit; Millipore) according to the manufacturer’s instructions. Normal goat serum (MP Biomedicals) was used as the substitution for the primary antibody for the negative controls.
Type II collagen mRNA expression in the chondrocytes was determined by fluorescent in situ hybridization. The sections were rehydrated after deparaffinization, permeabilized using 1% Triton X-100 in phosphate-buffered saline for 10 minutes, postfixed, and prehybridized. The slides were incubated overnight in a 50% formamide buffer (pH 7.0) at 52°C with a 1:400 diluted type II collagen antisense probe that was transcripted from a 435-bp cDNAErcoI-DraI fragment 17 and treated with RNase (10109134001; Roche Diagnostics, Mannheim, Germany) at 37°C for 10 minutes. The hybridized probe was visualized using a horseradish peroxidase conjugated anti-DIG antibody (11207733910; Roche Diagnostics), and the signal was amplified using a tyramide system (Molecular Probes, Eugene, Ore) as the manufacturer recommended. Vectashield mounting medium with DAPI (H-1200; Vector Labs, Burlingame, Calif) was used to seal the slides and distinguish the nuclei.
The toluidine blue, safranin-O, and immunohistochemistry stained sections were observed with an inverted microscope (Eclipse 80i; Nikon, Tokyo, Japan); the fluorescent in situ hybridization stained sections were observed using a laser confocal microscope system (TCS-SP2; Leica, Wetzlar, Germany), and the photographs were taken at 40-times magnification.
The microscopic photographs were analyzed using Image Pro Plus software (version 6.0.0.260; Media Cybernetics, Bethesda, Md). The suture width was measured by tracing the interface of the cartilaginous (metachromatic) and osseous tissues in the midpalatal suture. The cartilage area was defined as the metachromatic regions area on the toluidine blue and safranin-O stained sections; the chondrocyte area and the chondrocytes’ nuclei area were measured by tracing the areas of the chondrocytes and their nuclei, respectively. The chondrocyte numbers were counted, and their nucleus to cytoplasm ratio was defined as the chondrocytes’ nuclei area divided by the cytoplasm area (the chondrocyte area subtracted by the nuclei area). The PCNA and Ki-67 positive chondrocytes were counted, and the positive percentage was calculated vs the total chondrocyte count. The MMP-13 expression intensity in the chondrocytes was semiquantified using the “count and measure” tools of Image Pro Plus software, as previously described. All measurements were repeated 3 times, and the averaged values were used for the statistical analyses.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (version 19; SPSS, Chicago, Ill). The Kruskal-Wallis test was used to compare the differences in the groups, and the Bonferroni post hoc correction with a calibrated α was used for each paired comparison. The differences were considered statistically significant at P <0.05.
Results
The transient body weights decreased (8.2%) within 2 days after surgery, followed by a steady regain and final stabilization on day 7; this indicated that all animals tolerated the procedures well. No significant difference was found among the groups.
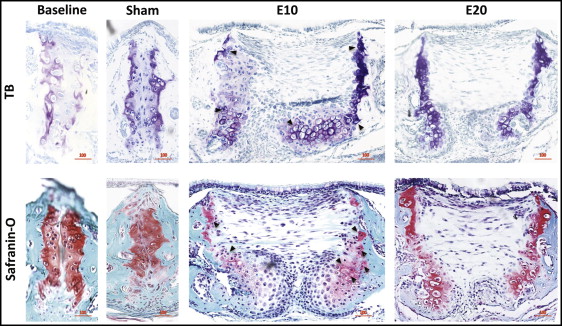
The baseline control group and the sham control group had few obvious differences for the toluidine blue or safranin-O staining ( Fig 2 ). Compared with the sham control group (154.8 μm), the 10-g expansion group had significantly widened sutures, to 936.0 μm, forming a pair of considerably cell-rich masses on the oral side, and the mitosis phase was observed in many chondrocytes ( Figs 2 and 3 ); the 20-g force group had expanded sutures to 870.4 μm without forming cell masses ( Fig 4 , A ).

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


