Introduction
In this study, we assessed the changes in bisphenol A (BPA) levels in saliva and urine after placing lingual bonded retainers.
Methods
Liquid chromatography/mass spectrometry was used to examine the BPA levels in the saliva and urine samples collected from 22 volunteers who received a lingual bonded retainer on their mandibular dentition. Samples were collected immediately before placement and 30 minutes, 1 day, 1 week, and 1 month after placement. The time elapsed after placement, type of resin composite (nanohybrid filled flowable resin or conventional hybrid resin), surface prophylaxis, age, and sex were evaluated for their effects on the BPA levels.
Results
The only significant high level of BPA was observed in the saliva collected just after placement of the lingual bonded retainer. Age and sex did not affect the BPA levels. Subjects in the flowable resin group had lower BPA levels than those in the conventional hybrid resin group; pumice prophylaxis decreased the level of BPA released from the conventional hybrid resin at the immediate time point. The salivary BPA level (maximum, 20.889 ng/mL) detected in the samples collected just after placement was far lower than the reference daily intake dose.
Conclusions
Accordingly, the potential toxicity of BPA from placing lingual bonded retainer might be negligible. On the other hand, because the health-effective amount of BPA is controversial, BPA release should be minimized.
Bisphenol A (BPA) is a well-known potent endocrine disruptor with a weak estrogenic effect. It is one of many commercial chemicals found in daily life, with more than 6.4 billion pounds produced worldwide in 2003 and growing at 6% to 10% per year. BPA has many applications in manufactured products, such as polycarbonate plastics, inner coating of food cans, antioxidants in cosmetics and food, and so on. In dentistry, BPA is a basic material for dental resins that is a precursor for BPA glycidyl dimethacrylate (Bis-GMA) and BPA dimethacrylate (Bis-DMA). On the other hand, the degradation of dental resins can leach BPA, and this reverse process is accelerated with heat, mechanical wear, and bacterial or salivary enzymatic action.
Articles on the possible hazardous effects of BPA have been published. BPA has an estrogenic effect, and the intake of BPA was reported to have a hazardous effect on living bodies. These effects involve increased growth rate, precocious puberty, decreased sperm count and infertility, increased risks of breast and prostate cancers, behavioral effects, and altered immune functions. Animal studies provided evidence of these effects, but the hazardous effects on human health are uncertain. Moreover, its risk assessment is unclear because the research design for humans is difficult. There is an extrapolated potential risk from animal study results, and it appears logical to err on the side of caution in this matter.
In dentistry, BPA is a source material used for manufacturing dental resins including dental sealants. Incomplete polymerization can result in the release of resin monomers into the oral cavity, and Bis-DMA–based resins have been shown to release BPA through biodegradation. Although Bis-GMA–based resins are reported not to release BPA from biodegradation, there is still the possibility that BPA and Bis-DMA, as impurities in the resins, might be released during biodegradation. Many studies have evaluated the amounts of BPA released from dental resins and attempted to assess the toxicologic effects of dental resins with in-vitro methods. Several studies reported significant releases of BPA from dental resins, whereas others reported no release or an insignificant release. These differences might be due to the different types of resins examined, different application methods of dental resins, and different analysis methods. More studies are needed to characterize BPA released from dental resins and its possible effects on human health.
An orthodontic lingual bonded retainer is frequently prescribed for patients at the end of orthodontic treatment to maintain the results of treatment. This remedy involves thin multi-stranded twisted wires bonded to the lingual surfaces of the teeth with resin composite that remain for several years to a lifetime in the oral cavity. A lingual bonded retainer is routinely prescribed to connect canine to canine or premolar to premolar, and a large amount of resin composite surface is exposed to the oral cavity. These resin composites are exposed to mastication forces and toothbrushing that cause mechanical wear, thermal changes from food and drink, and enzymatic attack from salivary and bacterial enzymes. In particular, for the mandibular anterior teeth, a lingual bonded retainer is a good harbor for dental calculus that attracts bacterial attachments. Although the lingual bonded retainer meets several conditions that facilitate biodegradation of the resin composite and has considerably large exposed surfaces and stays in the oral space for lengthy periods of time, there are no reports of BPA released from lingual bonded retainers by using in-vivo methods. The potential risk of BPA has attracted the attention of researchers and the public, and it appears that an evaluation of BPA leaching from resin composite will be inevitable. In this study, we examined the amounts of salivary and urinary BPA to assess the amount of BPA released from the resin composite used in a lingual bonded retainer during the first month of placement as part of a larger effort to confirm the safety of Bis-GMA–based dental composite resin.
Material and methods
Volunteers were recruited from patients undergoing orthodontic treatment who were planned to complete treatment between May 2009 and May 2010, and were scheduled to receive a lingual bonded retainer. The volunteers were excluded if they were smokers or had any general health disorders, such as renal or hepatic disease, and those whose occupation could be related to chronic heavy exposure to BPA, such as construction sites, gas stations, acryl related works, and so on. A total of 22 volunteers were included in this study: 10 male patients (mean age, 19.9 years; range, 13-25 years) and 12 female patients (mean age, 23.1 years; range, 13-32 years). A full explanation of the study objectives and protocols was given to the volunteers, and informed consent was obtained. The study protocol was approved by the ethical committee of Kyung Hee University Dental College (approval number KHUSD IRB 0906-01).
After removing the fixed orthodontic appliances, the patients were instructed to wear a vacuum-formed temporary retainer (polyvinylchloride, Essix A+, Dentsply, Bradenton, Fla) for a week. It was reported that the aerosol produced during the process of removing the orthodontic appliances contains resin monomers released from the resin composite used to bond the orthodontic brackets. Therefore, the inhalation of an aerosol by the patients might temporarily increase the BPA in their bodies. To avoid the effect of such a temporary increase, 1 week was allowed before collecting the samples. One week after removing the fixed orthodontic appliances, the patients received a lingual bonded retainer on the mandibular anterior dentition. For the maxillary dentition, a temporary retainer was worn through the experimental period. All patients received a lingual bonded retainer on their mandibular anterior teeth only. Some received a canine-to-canine lingual bonded retainer (total of 6 teeth), whereas others received a premolar-to-premolar (total of 8 teeth) lingual bonded retainer. One orthodontist (P.-J. W.) performed all bonding procedures to approximately standardize the amount of resin composite used to bond the lingual bonded retainer. Filtek Flow (Bis-GMA–based nanohybrid-filled flowable resin; 3M Unitek, Monrovia, Calif) or Z250 Universal Restorative (Bis-GMA based hybrid-filled restorative resin; 3M Unitek) were used to bond the lingual retainers. The specifications of the resin composites used in this study are given in Table I . The bonding procedure was performed according to the manufacturer’s recommendations. Briefly, the lingual surface of the teeth was cleaned with a pumice prophylaxis and then etched with Scotchbond etchant (3M ESPE, St Paul, Minn) for 15 seconds. After etching, a liquid adhesive resin (Adper Single Bond2, 3M ESPE) was applied and light-cured. Finally, Filtek Flow or Z250 Universal Restorative was applied and light-cured by using a plasma xenon arc light curing unit (wavelength, 450-500 nm; intensity, 1900 mw/cm 2 ; Flipo, Lokki, Les Roches de Condrieu, France). Light-curing was performed by radiating the occlusal and gingival surfaces of the resin for 3 seconds each. After bonding the retainer, pumice prophylaxis for each tooth’s resin surface or just water irrigation was performed for 5 seconds ( Table II ). The type of resin and the surface prophylaxis were assigned randomly.
| Flowable resin composite (Filtek Flow) | Hybrid resin composite (Filtek Z250) | |
|---|---|---|
| Composition | Bis-GMA (bisphenol A diglycidyl ether dimethacrylate) TEGDMA (tri[ethylene glycol] dimethacrylate) |
Bis-GMA (bisphenol A diglycidyl ether dimethacrylate) UDMA (urethane dimethacrylate) Bis-DEMA(6)1 (bisphenol A polyethylene glycol diether dimethacrylate) |
| Filler size | 0.01-6.0 μm (average, 1.5 μm) | 0.01-3.5 μm (average, 0.6 μm) |
| Filler content | 47% by volume (68% by weight) | 60% by volume (75% by weight) |
| Flowable resin composite (n = 10) | Hybrid resin composite (n = 12) | |
|---|---|---|
| Age (y) | 20.3 (5.8) | 23.5 (7.0) |
| Sex (female) | 7 (70%) | 5 (41.7%) |
| Pumice prophylaxis | 5 (50%) | 4 (33.3%) |
To evaluate the levels of BPA exposure, saliva and urine were collected before and after placing the lingual bonded retainer. Saliva was selected to measure the immediate release of BPA from the resin composite, and urine was selected to measure the amount of BPA absorbed by the body. First, saliva and urine samples were collected immediately before placing the lingual bonded retainer and used as the controls. A second saliva sample was collected 30 minutes after placing the retainer. Saliva and urine samples were collected after 1 day, 1 week, and 1 month. Table III lists the sample collection schedule. A total of 5 saliva samples and 4 urine samples were collected from each participant. All samples were stored immediately at −80°C until analysis. In addition, only glassware was used in the process involving sample collection and storage to prevent background contamination of BPA. Among the 22 volunteers, saliva samples from 2 volunteers were contaminated from breakage of the storage glassware. The total samples consisted of saliva samples from 20 volunteers and urine samples from 22 volunteers.
| Sampling time point | Saliva | Urine |
|---|---|---|
| Before bonding lingual retainer (control) | n = 20 | n = 22 |
| Immediately after bonding lingual bonded retainer | n = 20 | |
| 1 day after bonding lingual retainer | n = 19 | n = 22 |
| 1 week after bonding lingual retainer | n = 19 | n = 19 |
| 1 month after bonding lingual retainer | n = 20 | n = 22 |
Orally administered BPAs are absorbed from the gastrointestinal tract and undergo a first-pass effect in the liver and gut wall. The first-pass effect biotranforms the BPA to BPA-glucuronide or BPA-sulfate. Therefore, to determine the total BPA absorbed by the body, the conjugated BPA in the urine should be returned to its free form. To analyze the amount of total BPA, the urine samples were treated with glucuronidase and sulfatase (β-glucuronidase type H-2 from Helix pomatia, 10000 U/mL glucuronidase and 5000 U/mL sulfatase activity; Sigma, St Louis, Mo) for 90 minutes at 37°C before BPA analysis. In addition, the amount of BPA from the urine samples was normalized to the creatinine levels to account for the different urine volumes from the subjects. For high sensitivity and specificity, liquid chromatography/mass spectrometry was adopted as the analytical method for BPA.
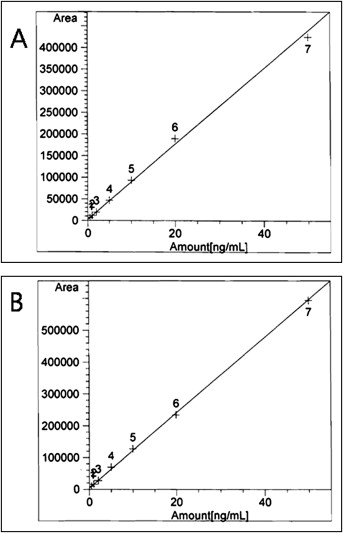
Standard solutions were prepared by diluting known amounts of BPA methanol solution at various concentrations in saliva or urine. A standard calibration curve was prepared by using the standard solutions. The standard calibration curve for BPA is given in Figure 1 .
The saliva and urine samples were thawed at room temperature before analysis. The thawed samples were then vortexed for 1 minute. Then we added 0.1 N of hydrogen chloride and 2 mL of methyl t-butyl ether to 500-μL aliquots of the sample and vortexed the samples again. The mixture was centrifuged at 3000 rpm for 5 minutes, and 1 mL of the supernatant was collected. The supernatant was evaporated to dryness in a nitrogen gas flow at 40°C. The dried residues were dissolved in 50 μL of methanol, and 10 μL was injected into the high performance liquid chromatography column.
A liquid chromatography/mass spectrometry system (1100 high performance liquid chromatography system; Agilent, Palo Alto, Calif) and a mass spectrometer (1956B; Agilent) were used to determine the levels of BPA. The resultant data were processed with ChemStation (Agilent). A Zorbax Eclipse XDB-C18 column (particle size, 5 μm; 4.6 × 150 mm; Agilent) was used. The mobile phase was a 60:40 (volume/volume) mixture of 0.1% acetic acid and acetonitrile. The flow rate was 0.7 mL per millimeter. The peak was detected in the selected ion monitoring mode, and the electrospray positive mode was used for ionization. Nitrogen gas was used as the nebulizing gas at a nebulizing pressure of 40 psi with a nebulizing gas flow rate of 12 L per minute, gas temperature of 300°C, and capillary voltage of 3500 V. The BPA signal was set to mass to charge ratio 227 with a 140-V fragment voltage. The limit of detection in liquid chromatography/mass spectrometry was 0.5 ng per millileter.
Statistical analysis
Various statistical methods were used to analyze the data collected from the 22 subjects. To investigate the difference in BPA between collection times, the random intercept models considering the correlation structure for collection times and the differences between subjects were used for the salivary and urinary BPA concentrations, respectively. In addition, the effects of the resin composite types, surface treatments, and sex differences were determined by using t tests assuming unequal variance, and the linear relationships between BPA and age were examined by using regression models. The relationship between the salivary BPA level immediately after resin placement and the urinary BPA 1 day after treatment was also evaluated by using the Pearson correlation coefficient. To analyze the patterns of the BPA concentrations for each subject across the collection times, a clustering method with the Euclidean distance and the Ward algorithm was used, and the clustering results were interpreted visually.
Results
Liquid chromatography/mass spectrometry showed that some saliva and urine samples contained BPA, and the descriptive analysis is presented in Table IV . The saliva samples collected immediately after lingual bonded retainer placement showed a significant increase in BPA compared with the baseline samples (before placing the lingual bonded retainer). However, the samples taken after 1 day, 1 week, and 1 month showed similar levels to the baseline ( Table IV ). All baseline saliva samples except for 2 showed undetectable (or below the quantification limit) levels of BPA, and 17 of 20 saliva samples collected immediately after retainer bonding contained BPA levels ranging from 0.853 to 20.889 ng per millileter. The urine samples contained BPA at various times but without an association with the time point ( Table IV ).
| Time point | Descriptive statistics (ng/mL) | Coefficients of times | SE | 95% CI | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Lower | Upper | |||||
| Saliva | Intercept | 0.4614 | 0.6409 | −0.8799 | 1.8027 | 0.4803 | |||
| Before | 0.461 | 1.701 | ND-7.497 | 0 | Baseline | ||||
| Immediately after | 5.042 | 5.734 | ND-20.889 | 4.5803 | 0.8746 | 2.8377 | 6.3229 | <0.0001 ∗ | |
| 1 day after | 0.446 | 1.337 | ND-4.42 | −0.0254 | 0.8867 | −1.7921 | 1.7413 | 0.9772 | |
| 1 week after | 0.491 | 1.700 | ND-7.395 | 0.0192 | 0.8867 | −1.7475 | 1.7859 | 0.9828 | |
| 1 month after | ND | 0.000 | ND-ND | −0.4614 | 0.8746 | −2.2040 | 1.2812 | 0.5994 | |
| Urine | Intercept | 0.5611 | 0.8844 | −1.2782 | 2.4003 | 0.5327 | |||
| Before | 0.561 | 1.262 | ND-5.415 | 0 | Baseline | ||||
| 1 day after | 2.351 | 4.780 | ND-22.327 | 1.7896 | 1.1521 | −0.5147 | 4.0941 | 0.1256 | |
| 1 week after | 2.083 | 4.687 | ND-20.390 | 1.5086 | 1.2018 | −0.8953 | 3.9125 | 0.2142 | |
| 1 month after | 1.805 | 3.143 | ND-12.767 | 1.2440 | 1.1521 | −1.0605 | 3.5484 | 0.2846 | |
The restorative Z250 resin composite produced higher BPA levels than did the flowable Filtek resin composite in the saliva sample only immediately after resin placement ( Table V ), and a higher BPA level was found in the water-irrigated samples than the pumice prophylaxis-treated samples only in the saliva samples collected immediately after Z250 resin placement ( Table VI ). Pumice prophylaxis did not affect the release of BPA in the flowable resin at all time points. Sex and age did not affect the BPA levels in the saliva and urine samples ( Tables VII and VIII ). The BPA level in the 1-day-after urine samples did not show an association with the BPA level in the saliva samples collected immediately after retainer placement.
| Time point | Resin | Sample number | Mean (ng/mL) | SD | 95% CI (F-Z) | P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Saliva | Before | F | 9 | ND | 0.0000 | −2.436 | 0.7577 | 0.2481 |
| Z | 11 | 0.8389 | 2.2685 | |||||
| Immediately after | F | 9 | 2.3211 | 2.2000 | −9.9420 | 0.0485 | 0.0420 ∗ | |
| Z | 11 | 7.2676 | 6.8186 | |||||
| 1 day after | F | 8 | 0.5525 | 1.5627 | −1.1610 | 1.5296 | 0.7860 | |
| Z | 11 | 0.3684 | 1.2217 | |||||
| 1 week after | F | 9 | 0.0914 | 0.2743 | −2.409 | 0.8918 | 0.3319 | |
| Z | 10 | 0.8502 | 2.3258 | |||||
| 1 month after | F | 9 | ND | 0.0000 | NA | NA | NA | |
| Z | 11 | ND | 0.0000 | |||||
| Urine | Before | F | 11 | 0.7974 | 1.6509 | −0.6570 | 0.6020 | 0.3979 |
| Z | 11 | 0.3284 | 0.7064 | |||||
| 1 day after | F | 11 | 0.5897 | 1.1459 | −7.5570 | 0.5128 | 0.0968 | |
| Z | 11 | 4.1116 | 6.3120 | |||||
| 1 week after | F | 9 | 0.6987 | 1.0267 | −7.1080 | 1.8468 | 0.2220 | |
| Z | 10 | 3.3291 | 6.2734 | |||||
| 1 month after | F | 11 | 2.8113 | 4.0100 | −0.6940 | 4.7193 | 0.1450 | |
| Z | 11 | 0.7988 | 1.5626 | |||||
| Time point | Resin | Surface treatment | Sample number | Mean | SD | 95% CI (O-X) | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Saliva | Before | F | X | 5 | 0.0000 | 0.0000 | NA | NA | NA |
| O | 4 | 0.0000 | 0.0000 | ||||||
| Z | X | 7 | 1.3183 | 2.7999 | −4.5600 | 1.9231 | 0.2593 | ||
| O | 4 | 0.0000 | 0.0000 | ||||||
| Immediately after | F | X | 5 | 2.2458 | 2.6910 | −3.5580 | 3.8971 | 0.9134 | |
| O | 4 | 2.4153 | 1.7973 | ||||||
| Z | X | 7 | 10.6280 | 6.3589 | −16.6800 | −1.8060 | 0.0079 ∗ | ||
| O | 4 | 1.3865 | 1.2802 | ||||||
| 1 day after | F | X | 4 | 1.1050 | 2.2100 | −3.8090 | 1.5988 | 0.3910 | |
| O | 4 | 0.0000 | 0.0000 | ||||||
| Z | X | 7 | 0.5789 | 1.5315 | −2.3520 | 1.1942 | 0.3559 | ||
| O | 4 | 0.0000 | 0.0000 | ||||||
| 1 week after | F | X | 5 | 0.1646 | 0.3681 | −0.6060 | 0.2767 | 0.3739 | |
| O | 4 | 0.0000 | 0.0000 | ||||||
| Z | X | 6 | 0.1845 | 0.4519 | −1.7480 | 5.0763 | 0.4352 | ||
| O | 4 | 1.8488 | 3.6975 | ||||||
| 1 month after | F | X | 5 | 0.0000 | 0.0000 | NA | NA | NA | |
| O | 4 | 0.0000 | 0.0000 | ||||||
| Z | X | 7 | 0.0000 | 0.0000 | NA | NA | NA | ||
| O | 4 | 0.0000 | 0.0000 | ||||||
| Urine | Before | F | X | 6 | 1.0350 | 2.1693 | −2.8740 | 1.8281 | 0.6057 |
| O | 5 | 0.5122 | 0.8630 | ||||||
| Z | X | 7 | 0.1816 | 0.3225 | −0.6190 | 1.4069 | 0.5474 | ||
| O | 4 | 0.5753 | 1.1506 | ||||||
| 1 day after | F | X | 6 | 0.2010 | 0.3187 | −0.6990 | 2.3789 | 0.3088 | |
| O | 5 | 1.0562 | 1.6300 | ||||||
| Z | X | 7 | 1.8688 | 1.4114 | −2.0400 | 14.3750 | 0.2987 | ||
| O | 4 | 8.0366 | 9.8255 | ||||||
| 1 week after | F | X | 5 | 0.7578 | 1.0736 | −1.8700 | 1.6039 | 0.8626 | |
| O | 4 | 0.6248 | 1.1229 | ||||||
| Z | X | 6 | 4.0841 | 8.0667 | −11.6700 | 7.8967 | 0.6119 | ||
| O | 4 | 2.1966 | 2.6006 | ||||||
| 1 month after | F | X | 6 | 3.2764 | 5.2540 | −6.7620 | 4.7151 | 0.6784 | |
| O | 5 | 2.2531 | 2.2317 | ||||||
| Z | X | 7 | 0.8358 | 1.9188 | −2.4360 | 2.2324 | 0.9070 | ||
| O | 4 | 0.7340 | 0.8756 | ||||||
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


