Phentolamine mesylate, at dosages from 0.4 to 0.8 mg in adults and adolescents and at dosages from 0.2 to 0.4 mg in children aged 4 to 11 years, has been proven to be safe and effective for the reversal of soft tissue anesthesia (lip and tongue numbness) and the associated functional deficits resulting from a local dental anesthetic injection containing a vasoconstrictor. Its ability to block a-adrenergic receptors on blood vessels induces vasodilation and enhances the redistribution of the local anesthetic away from the injection site. The low dosages administered for dental local anesthetic reversal in all likelihood accounts for the lack of significant cardiovascular effects that are associated with the medical use of the drug for hypertensive conditions associated with catecholamine excess.
Phentolamine mesylate, marketed under the proprietary name OraVerse, was granted Food and Drug Administration (FDA) approval on May 12, 2008 with an indication for the reversal of soft tissue anesthesia (lip and tongue numbness) and the associated functional deficits resulting from a local dental anesthetic containing a vasoconstrictor. This approved FDA indication does not include the use of this drug in children younger than 6 years or weighing less than 15 kg (33 lb) because of a lack of research in this younger age group. Phentolamine was originally developed for the treatment of hypertension and is still used off-label to treat hypertensive crises (characterized by catecholamine excess) by administering an intravenous bolus dose of 5 to 15 mg. Such hypertensive emergencies include drug (indirect sympathomimetics such as pseudoephedrine) and food interactions (those containing tyramine) with monoamine oxidase inhibitors, cocaine toxicity, and amphetamine overdose. The current FDA-approved intravenous and intramuscular formulation of phentolamine is indicated for the prevention and treatment of dermal necrosis resulting from the venous extravasation of the vasoconstrictor norepinephrine (which is used as a pressor agent in patients with severe hypotension) and for the diagnosis and treatment of severe hypertension in patients with pheochromocytoma (a rare tumor of the adrenal medulla that secretes excessive epinephrine and norepinephrine). Recommended intravenous and intramuscular dosages are 5 to 10 mg in adults and 1 to 3 mg in children. For dental local anesthetic reversal, the drug is packaged in a standard dental cartridge containing only 0.4 mg phentolamine mesylate in 1.7 mL of solution and is injected submucosally at the end of the dental procedure using the same location, volume, and injection technique as the previous local anesthetic injection.
Quality-of-life issues regarding lingering soft tissue anesthesia in dental patients
One of the shortcomings of dental local anesthetic agents, especially those used for routine restorative and scaling procedures that produce minimal postprocedural pain and are usually completed in less than an hour, is that the duration of soft tissue anesthesia (numbness to the lip and tongue) typically lasts for 3 to 5 hours. With mandibular block injections, lip anesthesia tends to persist longer than tongue anesthesia.
In adult dental patients, the major complaints concerning this residual anesthesia after leaving the dental office are difficulty speaking, smiling, drinking, and eating, plus a fear of drooling in public. Residual sensations of numbness after leaving the dental office have also been shown to provoke moderate to extreme emotions of dislike in the vast majority of adult and adolescent restorative patients. In children, prolonged soft tissue anesthesia can lead to inadvertent biting and mutilation of the lips, tongue, and cheeks. The results of one prospective study in 320 children revealed that 18% of children younger than 4 years, 16% of children between 4 and 7 years, and 13% of children between 8 and 11 years displayed postprocedural soft tissue trauma after receiving mandibular block injections. Lip biting injuries following local anesthetic injections are often misdiagnosed as bacterial infections and may result in unnecessary hospitalizations and unnecessary antibiotic administration.
Pharmacology of phentolamine
Phentolamine is classified as a nonselective α-adrenergic blocking agent, that is, it opposes the effects of norepinephrine and epinephrine on tissues containing α 1 – and α 2 -adrenergic receptors. In particular, the smooth muscles of many vascular beds, including those beneath the oral mucosa, contain α-receptors (predominantly α 1 ), and the ultimate effect of α-receptor blockade is vasodilation. At relatively high dosages (5–10 mg) that are used in conditions of local and systemic catecholamine excess, parenteral phentolamine produces a profound blood pressure lowering effect. This hypotensive action plus the ability of phentolamine to block prejunctional α 2 -autoreceptors on neuronal membranes can produce tachycardia both due to baroreceptor reflex mechanisms and an enhanced release of neuronal norepinephrine, which then stimulates β 1 -adrenergic receptors in the heart.
In contrast, oral submucosal injections of phentolamine at dosages that have been studied in phase 2 and 3 randomized, controlled clinical trials and subsequently approved by the FDA (0.2 mg–0.8 mg) have not been associated with significant cardiovascular changes, most notably hypotension or tachycardia, when compared with either placebo or sham injections. Previous to these trials, an open-label phase 1 study revealed that peak blood levels after administration of 1 cartridge of intravenous phentolamine alone (0.4 mg) was approximately 8 times higher than when the same dose was administered via an intraoral supraperiosteal infiltration injection over the maxillary first molar 30 minutes after a single cartridge of 2% lidocaine with 1:100,000 epinephrine was injected into the same site (10.98 vs 1.34 ng/mL). When 2 cartridges (0.8 mg or 3.4 mL) of intraoral phentolamine were administered into the same sites as 2 previous mandibular block and maxillary infiltration injections of lidocaine with epinephrine (4 cartridges or 7.2 mL total), an approximate doubling of peak phentolamine blood levels (2.73 ng/mL) was seen compared with the infiltration of a single phentolamine cartridge (1.34 ng/mL). From a safety standpoint, neither the submucosal doses nor the intravenous dose of phentolamine (which could be delivered clinically in the dental office via an inadvertent intravascular injection) produced hypotension at a rate greater than the infiltration administration of 4 cartridges of 2% lidocaine with 1:100,000 epinephrine alone. Because there are no published studies describing peak phentolamine blood levels after medical intravenous dosing (5–10 mg), direct pharmacokinetic comparisons with the intravenous or submucosal administration of only 0.4 to 0.8 mg used for dental local anesthetic reversal cannot be made. However, if it is assumed that the intravenous pharmacokinetics of phentolamine with respect to peak blood levels remains relatively linear, 5 to 10 mg of intravenous phentolamine would result in 12.5-fold greater blood levels (137 ng/mL–274 ng/mL) than that produced by the intravenous administration of 0.4 to 0.8 mg (11–22 ng/mL) and approximately 100-fold greater blood levels than 1 or 2 nonintravenous submucosal injections of 0.4 mg phentolamine (1.34–2.73 ng/mL). Thus, the low blood levels achieved with dental dosing, even when administered intravenously, in all likelihood accounted for the lack of untoward cardiovascular effects reported in this and subsequent clinical trials.
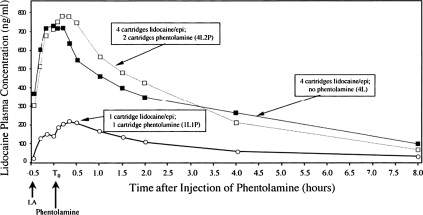
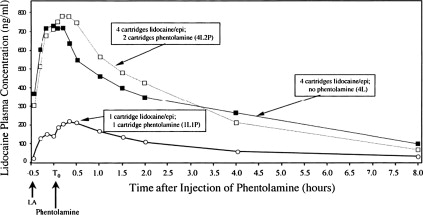
As illustrated in Fig. 1 , vasodilation induced by a subsequent phentolamine injection results in a more rapid redistribution of the local anesthetic away from the injection site compared with an injection of the local anesthetic solution alone. The lower concentration-time curve in this figure clearly shows a small but distinct second peak in lidocaine plasma concentrations immediately after the administration of 1 cartridge of phentolamine at the site of a previous lidocaine with epinephrine injection. Likewise, a second small peak was also produced when 4 cartridges of 2% lidocaine with 1:100,000 epinephrine was followed 30 minutes later by the injection of 2 cartridges of phentolamine into the same intraoral sites. In essence, the administration of phentolamine is antagonizing the α-adrenergic effects of epinephrine. A second peak in plasma levels was not observed when 4 cartridges of 2% lidocaine with 1:100,000 epinephrine was not followed by 2 phentolamine injections. From a safety standpoint, maximum plasma concentrations were only increased from 716.67 ng/mL with lidocaine plus epinephrine alone to 799.82 ng/mL when 4 cartridges of lidocaine with epinephrine were followed by phentolamine. This increase is still at least 5-fold less than lidocaine blood levels of 4500 ng/mL, which have been reported to be associated with early systemic local anesthetic toxicity.
Pharmacology of phentolamine
Phentolamine is classified as a nonselective α-adrenergic blocking agent, that is, it opposes the effects of norepinephrine and epinephrine on tissues containing α 1 – and α 2 -adrenergic receptors. In particular, the smooth muscles of many vascular beds, including those beneath the oral mucosa, contain α-receptors (predominantly α 1 ), and the ultimate effect of α-receptor blockade is vasodilation. At relatively high dosages (5–10 mg) that are used in conditions of local and systemic catecholamine excess, parenteral phentolamine produces a profound blood pressure lowering effect. This hypotensive action plus the ability of phentolamine to block prejunctional α 2 -autoreceptors on neuronal membranes can produce tachycardia both due to baroreceptor reflex mechanisms and an enhanced release of neuronal norepinephrine, which then stimulates β 1 -adrenergic receptors in the heart.
In contrast, oral submucosal injections of phentolamine at dosages that have been studied in phase 2 and 3 randomized, controlled clinical trials and subsequently approved by the FDA (0.2 mg–0.8 mg) have not been associated with significant cardiovascular changes, most notably hypotension or tachycardia, when compared with either placebo or sham injections. Previous to these trials, an open-label phase 1 study revealed that peak blood levels after administration of 1 cartridge of intravenous phentolamine alone (0.4 mg) was approximately 8 times higher than when the same dose was administered via an intraoral supraperiosteal infiltration injection over the maxillary first molar 30 minutes after a single cartridge of 2% lidocaine with 1:100,000 epinephrine was injected into the same site (10.98 vs 1.34 ng/mL). When 2 cartridges (0.8 mg or 3.4 mL) of intraoral phentolamine were administered into the same sites as 2 previous mandibular block and maxillary infiltration injections of lidocaine with epinephrine (4 cartridges or 7.2 mL total), an approximate doubling of peak phentolamine blood levels (2.73 ng/mL) was seen compared with the infiltration of a single phentolamine cartridge (1.34 ng/mL). From a safety standpoint, neither the submucosal doses nor the intravenous dose of phentolamine (which could be delivered clinically in the dental office via an inadvertent intravascular injection) produced hypotension at a rate greater than the infiltration administration of 4 cartridges of 2% lidocaine with 1:100,000 epinephrine alone. Because there are no published studies describing peak phentolamine blood levels after medical intravenous dosing (5–10 mg), direct pharmacokinetic comparisons with the intravenous or submucosal administration of only 0.4 to 0.8 mg used for dental local anesthetic reversal cannot be made. However, if it is assumed that the intravenous pharmacokinetics of phentolamine with respect to peak blood levels remains relatively linear, 5 to 10 mg of intravenous phentolamine would result in 12.5-fold greater blood levels (137 ng/mL–274 ng/mL) than that produced by the intravenous administration of 0.4 to 0.8 mg (11–22 ng/mL) and approximately 100-fold greater blood levels than 1 or 2 nonintravenous submucosal injections of 0.4 mg phentolamine (1.34–2.73 ng/mL). Thus, the low blood levels achieved with dental dosing, even when administered intravenously, in all likelihood accounted for the lack of untoward cardiovascular effects reported in this and subsequent clinical trials.
As illustrated in Fig. 1 , vasodilation induced by a subsequent phentolamine injection results in a more rapid redistribution of the local anesthetic away from the injection site compared with an injection of the local anesthetic solution alone. The lower concentration-time curve in this figure clearly shows a small but distinct second peak in lidocaine plasma concentrations immediately after the administration of 1 cartridge of phentolamine at the site of a previous lidocaine with epinephrine injection. Likewise, a second small peak was also produced when 4 cartridges of 2% lidocaine with 1:100,000 epinephrine was followed 30 minutes later by the injection of 2 cartridges of phentolamine into the same intraoral sites. In essence, the administration of phentolamine is antagonizing the α-adrenergic effects of epinephrine. A second peak in plasma levels was not observed when 4 cartridges of 2% lidocaine with 1:100,000 epinephrine was not followed by 2 phentolamine injections. From a safety standpoint, maximum plasma concentrations were only increased from 716.67 ng/mL with lidocaine plus epinephrine alone to 799.82 ng/mL when 4 cartridges of lidocaine with epinephrine were followed by phentolamine. This increase is still at least 5-fold less than lidocaine blood levels of 4500 ng/mL, which have been reported to be associated with early systemic local anesthetic toxicity.
Pivotal phase 2 and phase 3 efficacy and safety trials
The 4 pivotal clinical trials that are described involved healthy individuals in need of nonsurgical routine dental care consisting of simple restorative procedures, a single crown preparation, or a periodontal maintenance procedure requiring local anesthesia in combination with a vasoconstrictor in 1 quadrant of the mouth. The injection technique had to produce profound lip anesthesia and adequate pain control, which had to be achieved using 1 or 2 cartridges of local anesthetic. In all trials, bupivacaine with 1:200,000 epinephrine was not evaluated because its main use is to help control postsurgical pain via its prolonged duration of soft tissue and periosteal anesthesia.
Trial 1 was a phase 2, double-blind, randomized, placebo-controlled (using the phentolamine vehicle) study in 122 subjects between the ages of 10 and 65 years. Participants received 1 or 2 cartridges (as needed for pain control) of local anesthetic with vasoconstrictor before dental treatment in either the maxillary or mandibular arch. The local anesthetic solutions that were used included 2% lidocaine with 1:100,000 epinephrine, 4% articaine with 1:100,000 epinephrine, 4% prilocaine with 1:200,000 epinephrine, or 2% mepivacaine with 1:20,000 levonordefrin randomized in an equal allocation scheme. Immediately after treatment, an equal number of phentolamine 0.4 mg cartridges or phentolamine vehicle cartridges (to the previous local anesthetic volume) were injected in a 1:1 ratio. The primary efficacy end point was the median time to normal lip sensation recovery after the administration of study drug using a standardized lip tapping procedure, whereby lip sensation was rated as numb (no feeling), feeling pins and needles (tingling), or normal. For procedures performed in the lower arch, tongue and chin tapping procedures were used to evaluate sensory recovery of these tissues. Safety was assessed by recording spontaneously observed or reported adverse events, intraoperative Holter monitoring of cardiac rhythm, periodic recording of vital signs, and recording pain at the study drug injection site using a Heft-Parker 170-mm visual analog scale (VAS). The median time to the return of normal sensation of the lip in subjects who received phentolamine was 50 minutes in the maxillary arch and 101 minutes in the mandibular arch compared with 155 minutes and 150 minutes, respectively, in the placebo group ( P <.0001 in both arches in favor of phentolamine). This translated into a 68% acceleration in median recovery time in the maxillary arch and a 33% acceleration in median recovery time in the mandibular arch for lip anesthesia in the phentolamine group compared with placebo. The median time to recovery of normal sensation in the chin and tongue was also significantly more rapid ( P <.01) in the phentolamine group (80.5 and 73.5 minutes, respectively) than the placebo group (140 and 105 minutes, respectively). There were no serious adverse events observed in this study with a similar number of what was judged to be treatment-related adverse events reported in the phentolamine (n = 57) and placebo groups (n = 50). Most of these events were mild in severity. Tachycardia was the most frequently recorded adverse event, occurring in 22 individuals in the phentolamine group and 25 individuals in the placebo group. During the first 8 hours after study drug administration, the phentolamine-treated subjects reported an average pain rating of weak (30 and 32.5 mm) and the placebo-treated patients reported an average pain rating of faint (4 and 2 mm) in the maxilla and mandibular injection sites, respectively, as measured on the Heft-Parker VAS. Although these differences were statistically significant ( P <.05), they were not considered clinically meaningful and may have represented the more rapid dissipation of anesthesia in the phentolamine group.
Trials 2 and 3 were pivotal phase 3 trials in adults and adolescents with an age range of 12 to 92 years and used the same types of patients, local anesthetic and phentolamine dosages, and lip and tongue tapping sensory assessments as in trial 1. Lidocaine with epinephrine was administered to approximately two-thirds of the subjects, whereas the other one-third was divided among those receiving articaine with epinephrine, prilocaine with epinephrine, or mepivacaine with levonordefrin. Trial 2 (n = 244) was performed in the mandibular arch, and trial 3 (n = 240) was performed in the maxillary arch. Instead of using a placebo vehicle injection, a sham injection was used, whereby the plastic shield covering the local anesthetic needle was kept in place and pressed against the tissues by a single unblinded investigator at each research center, with a visual barrier covering the eyes of the research subjects. All subsequent measures of phentolamine or sham efficacy and safety were made by blinded investigators. In addition to the sensory assessments, subjects completed a soft tissue anesthesia recovery (STAR) questionnaire and a functional assessment battery (FAB) before the injection of local anesthetic, before the injection of phentolamine or sham, immediately after the injection of phentolamine or sham, and at various time points through 5 hours postadministration of phentolamine or sham. The STAR questionnaire measured the subject’s perception of altered function, sensation, and appearance. The FAB included measurements of smiling, speaking, presence or absence of drooling, and the ability to drink 3 ounces of water at various times during the study. A researcher and the subject rated each of these functional assessments as normal or abnormal. Safety measures included recordings of systolic and diastolic blood pressure and pulse and measurements of pain at various time points, including immediately after the local anesthetic injection and immediately after the sham or active phentolamine injection, using the Heft-Parker VAS.
The primary efficacy end point for trials 2 and 3 was the median time to recovery of normal lip sensation based on the subjects’ reports of numbness every 5 minutes while they performed the standardized lip palpation procedures. Secondary end points included median time to return to a score of zero derived from the STAR questionnaire; median time to return to normal on the FAB of smiling, speaking, drooling, and drinking; and, in the mandibular study (trial 2), the time to return of normal tongue sensation using the standardized tongue palpation method.
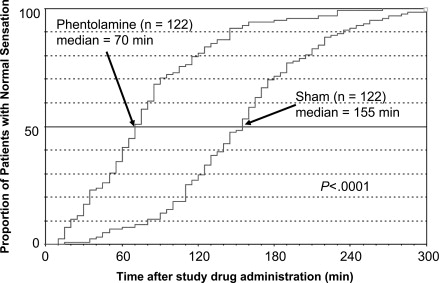
As displayed in Fig. 2 , the median time to recovery of normal lower lip sensation in trial 2 was 70 minutes (95% confidence interval [CI], 65–80 minutes) for subjects in the phentolamine group and 155 minutes (95% CI, 140–165 minutes) for subjects in the sham group ( P <.0001). The effect of phentolamine was an 85-minute reduction (54.8%) in median time to recovery of normal lower lip sensation. In addition, subjects in the phentolamine group were more likely to recover normal sensation in the lower lip during the early periods after injection of the study drug than were subjects in the sham group. Within the first 30 minutes, 21 subjects in the phentolamine group (17.2%) achieved normal lip sensation compared with only 1 subject in the sham group (0.8%). By 60 minutes, 50 subjects in the phentolamine group (40.9%) had achieved normal sensation compared with 9 subjects in the sham group (7.4%). By 90 minutes, 86 subjects in the phentolamine group (70.5%) compared with only 36 subjects in the sham group (13.1%) had achieved normal lip sensation. At 2 hours, 99 subjects in the phentolamine group (81.1%) and 36 subjects in the sham group (29.5%) had attained normal lip sensation. Only 23 subjects in the phentolamine group (18.9%) required more than 2 hours to achieve normal sensation in the lower lip compared with 86 subjects in the sham group (70.5%). Fig. 3 illustrates the return of normal tongue sensation in subjects in the mandibular study. The median times to recovery of normal sensation in the tongue were 60 and 125 minutes for subjects in the phentolamine and sham groups, respectively. The effect of phentolamine was a 65-minute reduction (52%) in median time to recovery of normal sensation for subjects in the phentolamine group compared with subjects in the sham group ( P <.0001). Subjects in the phentolamine group recovered at earlier times throughout the study. In addition, median recovery times on the STAR questionnaire and FAB were significantly more rapid ( P <.001) for this group than the sham group (90 and 60 minutes vs 150 and 120 minutes, respectively).