Alloplastic temporomandibular joint (TMJ) replacement is indicated as management of the following conditions:
- a.
Inflammatory arthritis involving the TMJ not responsive to other modalities of treatment
- b.
Recurrent fibrosis and/or bony ankylosis not responsive to other modalities of treatment
- c.
Failed tissue grafts (bone and soft tissue)
- d.
Failed alloplastic joint reconstruction
- e.
Loss of vertical mandibular height and/or occlusal relationship due to bony resorption, trauma, developmental abnormalities, or pathologic lesions.
At present, there are two types of alloplastic TMJ replacement devices available to the reconstructive surgeon: “stock” and patient-fitted (ie, “custom”). Both have fossa and ramus/condyle components, which are fixated and stabilized to the temporal bone and the lateral aspect of the ramus of the mandible, respectively, with bicortical screws.
The host fossa and ramus bone must be altered when implanting stock device components, or they must be bent or shimmed with bone or alloplastic cement to develop close host bone adaptation to the device components. Both of these maneuvers can lead to component material fatigue or overload, which promotes early failure under functional loading and, more concerning, allows micromotion of these altered or shimmed components that can interfere with osseointegration. Micromotion leads to the formation of a fibrous connective tissue interface between the altered component and the host bone, which results in early loosening of that component and potential early catastrophic or certain later premature device failure.
Because they are designed and manufactured for each specific anatomic situation, patient-fitted, or “custom,” alloplastic TMJ devices conform to any unique anatomic configuration, and require no significant host bone alteration or supplementation to achieve initial implantation stability. Because the components interface accurately with the host bone, the screw fixation secures the components to the host bone, mitigating any micromotion and maximizing the opportunity for osseointegration of the components and fixation screws; thus, there is greater potential longevity of “custom” devices under normal mandibular functional stresses.
This article provides the reader with an illustrated technique for placement of “custom” alloplastic TMJ replacement device components.
Preparation for surgery
Avoiding contamination of the surgical site is critical during any alloplastic TMJ replacement surgery; therefore, it is essential that complete sterility be maintained at the implantation sites throughout the procedure. The following patient preparation should be considered.
- •
The patient should be directed to thoroughly wash and rinse their hair the night before surgery with a mild shampoo and avoid the use of hair spray or styling gels the day of the surgery.
- •
As with any presurgical antibiotic prophylaxis regimen, intravenous antibiotics (eg, cefazolin 1 g, or clindamycin 600 mg) are begun 1 hour preoperatively and are maintained on an appropriate intravenous dosing schedule during the postoperative hospital course. This regimen is followed on discharge by 1 week of oral antibiotic (eg, cephradine 500 mg, clindamycin 300 mg) at the appropriate dosage.
- •
Anti-inflammatory steroid therapy to minimize edema may be started preincision (8–10 mg intravenous dexamethasone) and continued postoperatively as with other reconstruction or orthognathic surgery.
- •
Anesthesia may be delivered via a nasoendotracheal tube sutured to the nasal septum (2-0 silk), and the anesthesia tubing and equipment can be positioned near the patient’s feet. This arrangement allows for the draping that follows to decrease the potential for contamination and permits easier head movement in bilateral cases ( Fig. 1 ).
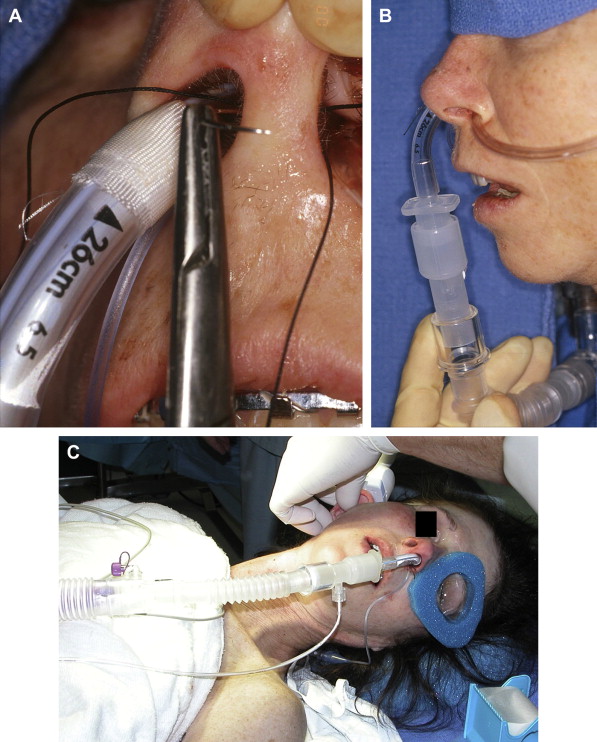
Fig. 1 ( A ) A 2-0 silk suture through nasal septum is tied around the nasoendotracheal tube to secure it in place during surgery. ( B ) Nasoendotracheal tube is secured to nasal septum with 2-0 silk suture and brought inferiorly away from the surgical sites. ( C ) Orientation of the anesthesia tubing and equipment toward the foot of the patient. - •
After the patient is anesthetized and the airway secured, the eyes should be lubricated and protected to prevent corneal abrasion ( Fig. 2 ).
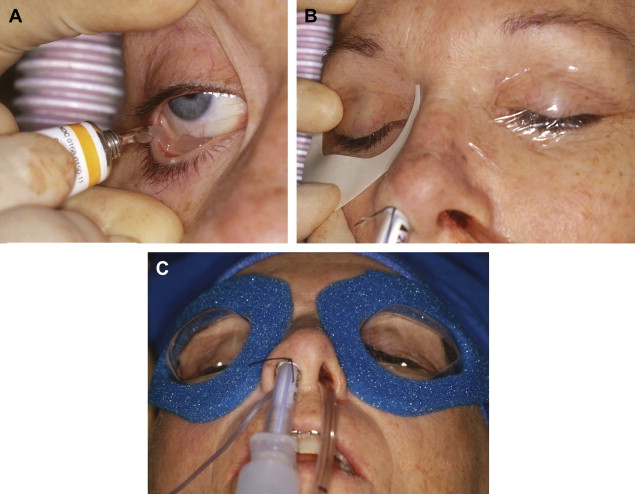
Fig. 2 ( A ) Lubrication of the eye. ( B ) Eyes taped shut. ( C ) Application of plastic goggles to protect the eyes during surgery. - •
Any hair that could become involved in the surgical field should be carefully arranged and/or parted to facilitate the skin incision. If the hair is to be sheared, care should be taken to avoid cutting or nicking the skin in the area of the surgical incision.
- •
After shearing the hair to above the ear, the remaining hair should be pulled away from the preauricular and surrounding areas and up toward the crown of the head.
- •
Using foam tape, the head should be wrapped circumferentially (forehead–above the ear–occiput) so that the hair is under the tape and off the skin over the preauricular incision site(s) ( Fig. 3 ).
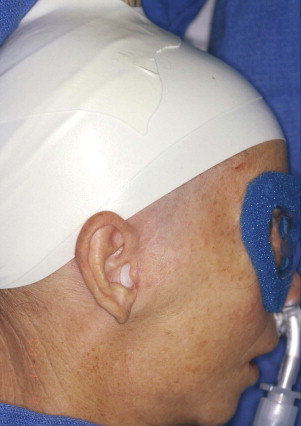
Fig. 3 The head is wrapped circumferentially with foam tape (forehead–above the ear–occiput) so that the hair is under the tape and off the skin over the preauricular incision site. Note the sterile mineral oil-cotton occlusive dressing in the external auditory canal. - •
The auditory canal(s) and tympanic membrane(s) should be inspected with an otoscope to ensure there is no preoperative infection and to document any presurgical pathology.
- •
The external auditory canal on the surgical side should be occluded. A cotton pledget moistened with sterile mineral oil is one option that can be utilized.
- •
Maxillomandibular fixation appliances (eg, arch bars, Ivy loops) should be applied prior to skin preparation and draping.
- •
All nonsterile maxillomandibular fixation instruments should be retained on a separate Mayo stand for use later in the procedure when the patient is placed in the final occlusion before device implantation.
- •
In unilateral cases, after appropriate skin preparation, a plastic adhesive isolation drape (eg, 1010 Steri-drape [3-M, St. Paul, MN, USA]) from the contralateral submental area to the ipsilateral temporal area isolates the mouth from the sterile surgical field. This type of draping allows access to the oral cavity while maintaining sterility of the implantation sites during application of intermaxillary fixation later in the procedure ( Fig. 4 ).
Fig. 4 Plastic adhesive isolation drape (eg, 1010 Steri-drape [3-M, St Paul, MN, USA]) from the contralateral submental area to the ipsilateral temporal area anterior to the surgical site isolates the surgical field from the nose and anesthesia equipment.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


