Fig. 12.1
Etiology of IANI and LNI [16]. The majority of IANI and LNI are caused by third molar surgery, followed by local anesthetics (LA). Only IANIs were also caused by implants and endodontics. IANI inferior alveolar nerve injuries, LNI lingual nerve injuries, TMS third molar surgery, LA local anesthetic injuries
Table 12.1
Management strategies for iatrogenic trigeminal nerve injuries [16]
|
Mechanism
|
Duration
|
Treatment
|
|---|---|---|
|
Known or suspected nerve transection
|
Immediate nerve exploration
|
|
|
TMS IANI – retained roots
|
<30 h
|
Immediate nerve exploration
|
|
Implant
|
<30 h
|
Remove implant
|
|
Implant
|
>30 h
|
Treat patient therapeutically
|
|
Endodontic
|
<30 h
|
Remove tooth/overfill
|
|
Endodontic
|
>30 h
|
Treat patient therapeutically
|
|
TMS IANI – large neuropathic area, pain, and disability
|
<3 months
|
Consider nerve exploration
|
|
TMS LNI – large neuropathic area, pain, and disability
|
<3 months
|
Consider nerve exploration
|
|
TMS IANI
|
>6 month
|
Treat patient therapeutically
|
|
TMS LNI
|
>6 month
|
Treat patient therapeutically
|
|
Local anesthesia, jaw fracture, orthognathic, other surgery
|
Treat patient therapeutically
|
High-dose corticosteroids and/or nonsteroidal anti-inflammatory medications administered in the early days following nerve injury should reduce local inflammation and, in theory, should minimize further damage to the injured nerve, but paradoxically these medications could interfere with the neural healing process. To date, there is little or no evidence that this pharmacologic intervention will minimize the extent and duration of trigeminal nerve injury [20].
The clinician responsible for the nerve injury must be honest and caring with the patient and show concern with a home check, or a phone call to the patient within 6–24 h post-surgery, to ensure that the clinician knows if there is any extreme pain or neuropathy that may be associated with the nerve injury and avail the patient of the appropriate intervention options, if required. Poor management by the clinician, extreme defensive behavior, and/or ignoring the patient’s complaints will only add to the frustration and anger of the patient, compounding the injured patients’ experience, so these injuries must be managed empathetically.
12.2.2 Later or Delayed Management
Later or delayed management of nerve injuries will depend upon the mechanism and the duration of the event (Table 12.1) [16]. The patient’s ability to cope with the neuropathy and pain, functional problems, and their overall psychological status will drive the need for therapeutic intervention. Considering that the majority of these patients present with neuropathic pain, most are managed with reassurance and medications; however, psychological techniques are being developed for these patients. Many injuries have limited benefit from surgical intervention and should be managed symptomatically using medication or counseling. In order to manage the patient appropriately, you must assess what is causing the patient’s problems. It is important to identify key symptoms including pain or altered sensory perception that may be impairing the patient’s functional abilities. Secondly, it is critical to inquire about functional problems (Fig. 12.2) in order to identify what specifically is most responsible for the patient’s distress. Symptoms like numbness with pain with light touch (mechanical allodynia) or pain with cold stimuli (cold allodynia) often confuses and distresses the patient. An explanation of these symptoms by the clinician often alleviates the patient’s anxiety in most cases. Therapeutic interventions may include the following:
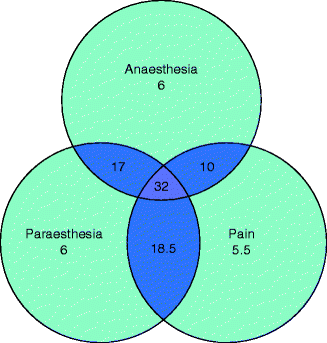

Fig. 12.2
Incidence (%) of pain, anesthesia, and paresthesia among all patients [16]
1.
Consultation, reassurance, and understanding will assist many patients in dealing with these nerve injuries. Education about their condition and reassurance that the damaged nerves will not lead to more serious diseases probably is the first and most powerful intervention for the patient. Psychological intervention is recommended for irreversible injuries and injuries that cannot be surgically rectified (e.g., LA-related, endodontic chemical damage, and gross surgery) in patients who are challenged by coming to terms with the permanent nonoperative nerve injury.
2.
Medical pharmacologic symptomatic therapy may be indicated for patients with pain or discomfort. Medications for chronic pain may include:
-
Topical agents for pain
-
Systemic agents for pain
3.
Surgical exploration (as discussed in other chapters)
-
Immediate repair if nerve transection is known or suspected
-
Removal of an implant or endodontic filling material within 30 h
-
Exploration of IAN injuries through the extraction socket (less than 4 weeks)
-
Exploration of LN injuries before 12 weeks
The surgical management for trigeminal nerve injuries is discussed in the other chapters of this book, and this chapter addresses several nonsurgical strategies that can be used to assist the practitioner in preventing and managing complications related to some common dental surgical procedures. In a recent study, surgery with no other form of treatment was a management option for only 22 % of all patients presenting with trigeminal nerve injuries [16].
The planned treatment must address the patient’s concerns appropriately, and the aims of treatment would ideally include the reduction of pain and discomfort and ultimately provide improved neurosensory function. It is important to stress that treatment may not completely restore function, such as eating, drinking, speaking, and sleeping; in addition, any treatment will not restore normal sensation in the neuropathic area, including general sensory (i.e., mechanosensory function) or special sensory function (i.e., taste). Escalation of a patient’s symptoms from intermittent pain to persistent pain would be a significant negative outcome, as would causing a patient to have discomfort or pain when previously they only reported anesthesia. Therefore, particularly with surgery, each patient must be warned of the potential risk of escalating their neuropathic symptoms, which in this study [16] resulted in 40 % of patients declining offered reparative nerve surgery.
12.3 Nonsurgical Management of Trigeminal Nerve Injuries
The strategy for selecting the mode and timing of intervention must be based upon the etiology of the injury, the patient’s current symptoms, the extent and permanency of the injury, and ultimately the patients’ choice of treatment following informed consent and education by the clinician. The key management strategies include counseling and reassurance, medication, and surgery.
In order to successfully manage patients with nerve injuries, the clinician must consult with the patient in an in-depth fashion, provide realistic expectations by reaffirming the nerve injury is permanent if the patient has had their symptoms for more than 3 months, and provide reassurance that these injuries do not predispose them to other disease processes (e.g., cancer), and indeed will likely not worsen. Such reassurance can successfully manage patients who can manage their pain but cannot cope with the consequences of their nerve injury and have associated functional chronic neuropathic pain and resultant psychological difficulties that significantly impact upon their social life or professional life, or usual activities of daily living.
12.3.1 Functional Morbidity
Despite recognition that lingual nerve injuries can cause significant deficiencies in pronunciation [21], there is no evidence that patients suffering with speech problems due to their nerve injury may also benefit from speech therapy. A patient with a nerve injury that complains of disability associated with altered sensation, severe discomfort, pain and/or numbness, or a large neuropathic area may also complain of interference with daily functions, such as eating and drinking (Fig. 12.2) [3]. Taste is also a function that may be impaired with some lingual nerve injuries due to involvement of the chorda tympani branch of the facial nerve as it courses with the lingual nerve. Inability to perform functional activities of daily life, such as applying lipstick, toothbrushing, kissing, or shaving due to an inferior alveolar nerve injury, may also occur. Also, sleep patterns may be affected (Fig. 12.3).
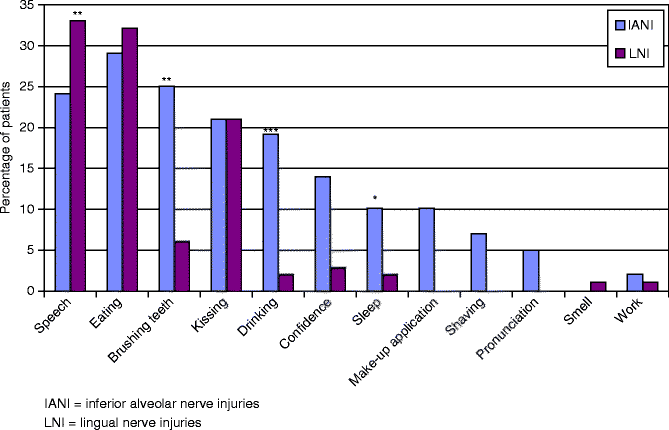

Fig. 12.3
Interference with functionality of the IANI and LNI patients. The majority of IANI and LNI patients had problems with speech and eating, where speech was significantly affected more in lNI patients than IANI patients. (**p<.001) drinking (***p<.0001) confidence (**p<.001), and sleep (*p<.05) [3]
12.3.2 Pain Management
Neuropathic pain is defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” [11]. Unlike nociceptive pain, neuropathy is associated with “shooting” or “burning” pains, sensations similar to electric shocks, and abnormal responses to touch, heat, or cold. This type of pain typically does not respond to anti-inflammatory analgesics.
Neuropathic pain is reported to be present in 50–70 % of patients attending specialist nerve injury clinics [3, 16, 22]. Despite the additional presence of anesthesia and/or paresthesia, a similar cohort of patients was reported to have a 45 % incidence of dysesthesia (Robinson, JOMS 2011). Neuropathy was evident in all patients with varying degrees of mechanosensory functional loss, paresthesia, dysesthesia, allodynia, and hyperalgesia. Patients with chronic neuropathy were treated by one or more of the following three key modalities: counseling, medical intervention usually for pain (antiepileptics or antidepressants), the application of topical 5 % lidocaine patches, and/or lastly surgery (Table 12.2) [11]. In order to make the correct choice of management of patients with nerve injury, the clinician must discern what he/she is attempting to treat; is it poor mechanosensory function, or, more pertinently, should it be the patient’s chief complaint? Therefore, a thorough assessment of the patient is vital before making such an important decision.
Table 12.2
Medical management of neuropathic pain [11]
|
Drug
|
Starting dose
|
Maximum dose
|
Cost of 4 week treatment
|
Comments
|
|---|---|---|---|---|
|
Oral agents: refer to product literature for full list of doses, cautions, contraindications and drug interactions
|
||||
|
Amitriptyline
|
10 mg/day
|
75 mg/day
|
10 mg/day = £1.12
|
Higher doses could be considered in consultation with a specialist pain service
|
|
25 mg/day = £1.13
|
||||
|
75 mg/day = £2.39
|
||||
|
Duloxetine
|
60 mg/day
|
120 mg/day
|
60 mg/day = £27.72
|
Maximum of 120mg daily in divided doses
|
|
120 mg/day = £55.44
|
||||
|
Gabapentin
|
300 mg/day (see comments)
|
3.6 g/day
|
900 mg/day = £4.19
|
300 mg once daily on day 1, then 300 mg twice daily on day 2, then 300 mg three times daily (approx. every 8 h) on day 3 or initially 300 mg 3 times daily on day 1, then increased according to response in steps of 300 mg daily (in three divided doses) every 2–3 days to max. 3.6 g
|
|
1.8 g/day = £8.38
|
||||
|
2.7 g/day = £12.57
|
||||
|
3.6 g/day = £15.12
|
The titration above has been recommended by the BNF 59. Local expert opinion suggests that a slower titration than the BNF recommendation may improve tolerance to gabapentin
|
|||
|
Pregabalin
|
150 mg/day in two divided dosed
|
600 mg/day
|
150 mg/day = £64.40
|
A lower starting dose may be appropriate for some
|
|
200 mg/day = £64.40
|
||||
|
300 mg/day = £64.40
|
||||
|
600 mg/day = £64.40
|
||||
|
Tramadol
|
50–100 mg not more often than every 4 h
|
400 mg/day
|
400 mg/day = £6.38
|
There is a possible interaction between duloxetine and tramadol: possible increased serotonergic effects when duloxetine given with tramadol—use with caution
|
|
Prices based on the Drug Tariff August 2010
|
||||
|
Full range of doses not listed under cost. Costs based on 4 week treatment and is stated for information purposes only
|
||||
|
Topical agents: refer to product literature for full list of doses, cautions, contraindications and drug interactions
|
||||
|
Lidocaine Patch
|
Apply patch to skin once daily for up to 12 h followed by a 12 h plaster free period
|
Up to 3 plasters may be used to cover large areas
|
1 patch daily = £67.57
|
Apply to intact, dry, non-hairy, non-irritated skin once daily for up to 12 h, followed by a 12-h plaster-free period; discontinue if no response after 4 weeks
|
|
3 patch daily = £202.72
|
Up to 3 plasters may be used to cover large areas; plasters may be cut
|
|||
|
Prices based on the BNF 59 March 2010
|
||||
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


