Two phases
Early phase: constant vs. moving touch
Late phase: directionality
Frequency
Three or four times a day for a couple of minutes
General strategies
1. Quiet surroundings
2. Concentration is important
3. Use stimulus (cloth, cosmetic brush, cotton swab), not finger
4. Using a finger would create two sets of sensory information for the patient which would confuse the already distorted sensory picture
Components of retraining
1. Observation of touch/movement. For the face, it’s critical to use visual feedback via a mirror
2. Concentration on perception of touch/movement with eyes closed in order to combine the mental with the visual picture
3. Repeat observation for visual confirmation of touch/movement
4. Verbalize the touch/movement being performed and what it feels like
5. Incorporate unaffected areas using the same procedure so that the sensation on the two sides may be compared
The process of sensory retraining can be thought of as similar to the brain learning a new language with progressive, increasing phases of difficulty. Initially when learning a language, use of words is slow, challenging, and error prone. With time and practice, language fluency may be acquired. Unfortunately, no research has been conducted to determine the optimal number of phases or the exercises required to obtain the maximum benefit to patients with orofacial nerve injuries. The potential to acquire the “second language” by sensory retraining does decrease with age [51, 62, 63], varies with the verbal learning capacity and visuospatial cognitive skills of the patient, and depends on motivation and positive reinforcement [50].
18.10 Sensory Retraining After Injuries to the Orofacial Region
18.10.1 Initial Clinical Observations
The question of whether sensory retraining exercises could be used effectively with patients with altered orofacial sensation was first raised in the 1992 literature by Gregg [32]. In 2001, Meyer and Rath presented a retrospective review of 372 patients who had had a microsurgical repair for a nerve injury after 1981 and for whom at least an 18-month postsurgical follow-up was available. A nonrandom sample of patients had been given facial sensory exercise instructions that incorporated some of the early-stage components of sensory retraining with the expectation that sensory retraining would help patients with altered orofacial sensation following nerve injury by (1) improving patients’ ability to interpret lip/chin sensations and movements, (2) improving perioral motor function subjectively and objectively, and (3) lessening the objectionable impression of numb/paresthetic sensations in the lip and chin by decreasing the subjective differences between affected and unaffected skin areas [55]. The percentage of patients who achieved a useful sensory recovery on the Medical Research Council Scale (MRCS), a clinical assessment, did not differ for those who did and did not receive instructions regarding facial sensory exercises. However, those patients who received instructions reached their final level of sensory recovery much sooner, on average 3 months earlier [55].
18.10.2 Findings from a Randomized Clinical Trial
In order to assess the efficacy of sensory retraining for facial altered sensation, a multicenter double-blind parallel two-arm stratified block randomized clinical trial (RCT) was conducted at an academic center and a community-based center with enrollment of 191 subjects [61]. The intent was to assess whether the magnitude and duration of patient-reported burden from altered sensation was lessened when facial sensory retraining exercises were performed in conjunction with standard opening exercises than when the opening exercises were performed alone. The subjects were patients with a developmental disharmony who were scheduled for bilateral sagittal split osteotomy with or without maxillary osteotomy.
The emphasis in the RCT on patient-report was motivated by two factors: (1) the assumption that sensory retraining would not affect actual nerve recovery including objective sensory testing measures of nerve function and (2) the recognition of the difference in function of the sensory innervations to the facial as compared to skin in other anatomic areas like the fingers. The terminal distribution of the inferior alveolar nerve, i.e., the mental nerve, innervates skin functionally more like the back of the hand (radial nerve) than the palm side of the hand (median and ulnar nerves) [16, 84]. Thus, the receptors in the skin of the hairy lower lip/chin of the face deform in response to movements during function, and as such, the evoked neural discharge serves a proprioceptive role including a conscious awareness of facial expressions [16, 30].
The sensory retraining protocol in the RCT had three time-dependent levels of instructions that were given to patients at 1 week, 1 month (4–6 weeks), and 3 months after surgery. The time points were selected based on their use in clinical studies of the impact of sensory reeducation in patients with an injured median or ulnar nerve [94] and in clinical studies of sensory impairment in patients following orthognathic surgery [29, 48, 85, 96]. The three levels of sensory retraining were designed to increasingly challenge patients congruent with the early and late phases of sensory education used for the hand: constant versus moving touch, orientation of moving touch, and direction of moving touch (Table 18.2). (Three videos demonstrating each exercise at each level are available online at http://www.oralmaxsurgeryatlas.theclinics.com/article/S1061-3315(10)00065-X/abstract, March 2011 issue. The videos were produced by Video Services of the Center for Instructional Technology at the University of North Carolina. Written instructions provided to subjects and copies of the instructional tapes are available from the corresponding author upon request.)
Table 18.2
Synopsis of instructions for sensory reeducation training
|
Visit
|
Sensory retraining exercises
|
|---|---|
|
1 week
|
Alternate simple touch and stroke with cosmetic brush (motion training)
|
|
Feedback from mirror
|
|
|
Visualization with eyes closed
|
|
|
1 month
|
Alternate up/down and side/side strokes (orientation training)
|
|
Feedback from mirror
|
|
|
Visualization with eyes closed
|
|
|
3 months
|
Alternate up → down and down → up strokes (directionality training)
|
|
Feedback from mirror
|
|
|
Visualization with eyes closed
|
Consistent with the anecdotal reports, the patients in this clinical trial who received the sensory retraining exercise instruction were less likely to report a problem related to unusual feelings on the face, loss of lip sensitivity, or numbness at 3 and 6 months after surgery than subjects who received standard opening exercises only [61]. At 6 months, subjects in the opening-only exercise group were almost twice as likely as those in the sensory retraining group to report a problem with altered sensation [61]. In addition to patient-reported outcomes, two-point perception, two-point discrimination, and contact detection thresholds were measured as secondary outcomes. The sensory retraining patients were more adept at discerning touch, indicating accommodation, even though there was no improvement in the ability to discriminate two distinct points of contact from one or to detect the presence of tactile stimulation (nerve recovery) [22].
The positive effect of the sensory retraining persisted even after the exercise protocol was completed (Fig. 18.1). Although the likelihood that a subject would report altered sensation steadily decreased in both groups over a 2-year follow-up protocol, the difference between the groups was relatively constant. Even at 2 years after surgery, patients who received only the opening exercises were about two times more likely to report an altered sensation than patients who used the sensory retraining exercises after surgery (Fig. 18.1) [62]. And patients in the sensory retraining group were less likely to report interference in daily life activities from numbness or loss of lip sensitivity (Fig. 18.2a, b) [63]. This difference between the two exercise groups appears to be related to the difference in how the “retrained” individual experienced or interpreted tactile stimuli rather than any difference in nerve recovery or repair [22, 23]. Two years after surgery, patients who received sensory training during the initial 3-month period still exhibited greater sensitivity on tests of two-point perception, i.e., sensory retraining subjects reported perceiving two-point contact from a one-point contact at a shorter separation between the points (Fig. 18.3) [23].
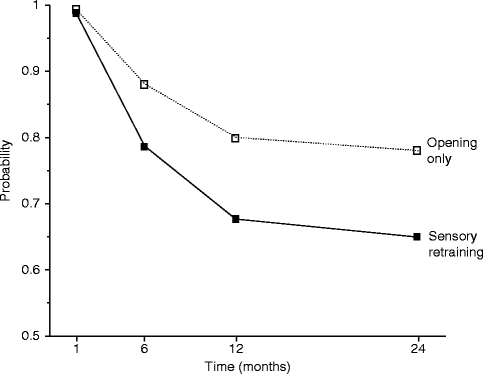
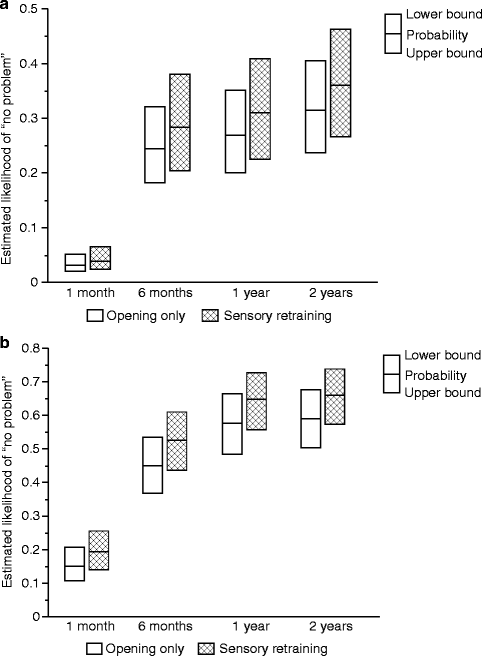
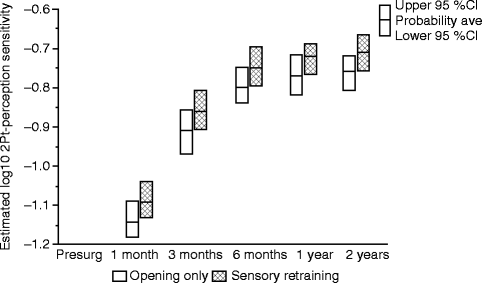

Fig. 18.1
Estimated likelihood of the presence of altered sensations for subjects who did and did not receive sensory retraining exercises after controlling for psychological distress and age (Data from Phillips et al. [62])

Fig. 18.2
Estimated likelihood of a subject reporting no problem or interference in daily life after controlling for psychological distress and age for subjects who did and did not receive sensory retraining exercises. (a) No problem associated with numbness. (b) No problem associated with loss of lip sensitivity (Data from Phillips et al. [63])

Fig. 18.3
Estimates of the adjusted mean sensitivity in two-point perception for subjects who did and did not receive sensory retraining exercises. The higher two-point perception sensitivity, on average, for the sensory retraining (SR) group indicates that this group was able to report two distinct points at separations closer to that reported before surgery than the opening-only group (Data from Essick et al. [23])
The results at 6 months and the longer-term analyses at 24 months suggest that for patients who experience an acute nerve injury, as is highly likely during a mandibular osteotomy, the simple, noninvasive sensory retraining facial exercises, which require only an inexpensive cosmetic brush and a mirror, are an effective therapy to promote accommodation to a sensory deficit on the face. Perhaps the desired outcome for “retrained” patients was best stated by Callahan, “If sensory re-education results in a person’s increased ability to better enjoy the tactile sensations of everyday living, then reeducation has been meaningful and successful” [9].
For orofacial sensory retraining, an important component of the retraining is the visual feedback provided by performing the exercises in front of a mirror. This elicits two different sensory events, the sensation of the brush on the facial skin and the sight of the brush on the face. Recent experimental studies have shown that viewing a body surface can directly enhance tactile perception and detection [27, 79] even when the “touch” is not physical but a mirrored reflection [71, 74]. The frequency of the repetitiveness with which the exercises are performed each day is much more important than the length of time spent at any given point in time. It may be that encouraging patients to perform orofacial sensory retraining exercises with a small handheld mirror for a short period of time, perhaps 1–2 min, four to six times per day would be more effective than a longer time in a less frequent protocol.
18.11 Vitamin B12 Alters the Peripheral Neural Response to Nerve Injury
Vitamin B12 is a potential pharmacological treatment for the restoration of nerve function after acute peripheral nerve injury. The neurodegenerative effects of vitamin B12 deficiency and the resolution of these effects with vitamin B12 supplementation are well documented [35, 76]. Vitamin B12 supplementation has been suggested as an ameliorative treatment for conditions such as amyotrophic lateral sclerosis, diabetic neuropathy, Bell’s palsy, carpal tunnel syndrome, and dialysis-induced neuropathy [37, 41, 47, 49, 73, 75, 78, 95]. These studies involving patients with chronic conditions associated with accompanying peripheral nerve damage indicate that vitamin B12 supplementation to therapeutic levels improves nerve function with a decrease in pain.
In vivo studies in non-B12-deficient animal models have shown that vitamin B12 treatment after acute peripheral nerve injury increases the production of neurotrophic factors that impede nerve degeneration and promotes nerve regeneration [43, 56, 89, 90]. In addition to the experimental animal peripheral nerve injury models [43, 56, 89, 90], an open randomized control trial reported shorter recovery times from Bell’s palsy with intramuscular methylcobalamin or methylcobalamin plus prednisone (ca 2 weeks) than with prednisone alone (ca 10 weeks) [41]. However, clinical studies to assess the effect of vitamin B12 supplementation on sensory nerve recovery after acute peripheral nerve injury in otherwise healthy human subjects are limited [65, 66].
18.12 Vitamin B12 After Injuries to the Orofacial Region
Unlike sensory retraining, vitamin B12 was expected to have an effect on the restoration of peripheral nerve function. For this reason, contact detection and thermal threshold were used as objective primary outcomes in our two exploratory B12 studies.
18.13 Intranasal Vitamin B12 Is Well Tolerated
Initially, we demonstrated the tolerability of weekly intranasal vitamin B12 spray applications beginning 2–3 weeks before surgery and continuing for 6 months after surgery in healthy, non-B12-deficient patients having orthognathic surgery with nasal intubation. The total serum cyanocobalamin levels reached therapeutic levels (above normal range) in the majority of patients by 1 month after surgery. A particularly encouraging finding of the tolerability study was that those patients who exhibited higher increases in B12 serum levels from baseline were less impaired in touch detection (a large diameter nerve fiber function) than those patients who exhibited lower increases in B12 levels [65].
18.14 Intranasal Vitamin B12 Alters the Course of Sensory Impairment
Subsequently, pilot data on the effect of B12 on small diameter (thermal thresholds) as well as large diameter nerve fiber functions were obtained in an exploratory randomized clinical trial comparing the sensory impairment following orthognathic surgery of intranasal B12 spray administered in conjunction with sensory retraining exercises compared to sensory retraining alone. Sensory retraining does not alter the course of nerve regeneration or the absolute thresholds to touch at the injured nerve site [5, 22, 23, 45], but only improves the patient’s cognitive and adaptive response to stimulation of the affected skin region [14, 61, 62, 64]. For these reasons, we reasoned that the combination of vitamin B12 and sensory retraining might provide a noninvasive means for patients to achieve optimal recovery of sensory function following trigeminal nerve injury.
Although only a small trial, a parallel randomized block design was used, and the two treatment groups (vitamin B12 with sensory retraining vs. sensory retraining only) were quite similar at enrollment with respect to sex, type of surgery, and average ages at the time of surgery. The average threshold values of the two groups did not differ significantly for any of the threshold measures before surgery, i.e., before the initiation of any therapy [66]. At the time of surgery (approximately 3 weeks after initiation of the intranasal B12 spray in the SR + B12 group) and at 6 months after surgery, the average serum B12 levels were significantly different for the two groups, while the average change in serum B12 levels from surgery to 6 months was not statistically significant in either group (Fig. 18.4). As expected, the average serum B12 levels were substantially higher at both visits in the SR + B12 group, approximately 40 % higher at the time of surgery and 35 % higher at 6 months. This indicates that the increase in B12 levels in the SR + B12 group had been reached by the time of surgery and then was maintained during the postsurgery time frame.
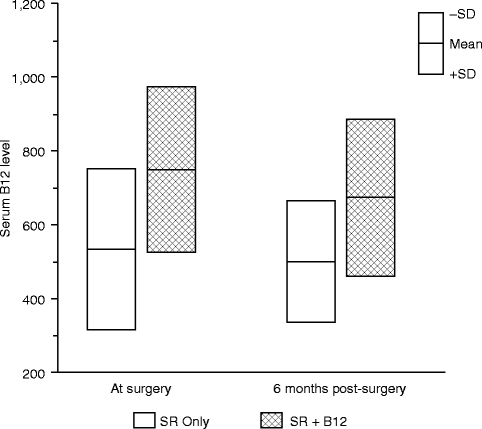

Fig. 18.4
Comparison of the serum B12 level in the two treatment groups at the time of surgery and 6 months postsurgery (Data from Phillips et al. [66])
18.15 Contact Detection
Since vitamin B12 supplementation for vitamin B12 deficiency appears to impact both axon and myelin structures of large diameter Aβ nerve fibers [2, 26, 67], we hypothesized that the CDTs would be less impaired in the SR + B12 group than in the SR-only group. Both the data of this study and the previous study [65] support this hypothesis. In the B12 tolerability study, patients who experienced greater increases in B12 serum levels (>400 pg/ml) at the time of postsurgical tactile testing compared to the presurgical baseline levels exhibited less impairment in touch detection associated with the mandibular osteotomies than patients who experienced lesser increases in B12 serum levels. The data from the exploratory RCT additionally indicated that the largest difference between groups occurred early (1 month) after nerve injury (Fig. 18.5). The better tactile detection sensitivity at 1-month postsurgery in the SR + B12 group may indicate faster remyelination in the SR + B12 group than in the SR-only group since a higher proportion of traumatically injured nerves likely experience demyelination damage than partial axonal laceration damage and recovery from demyelination occurs more quickly [40, 83].
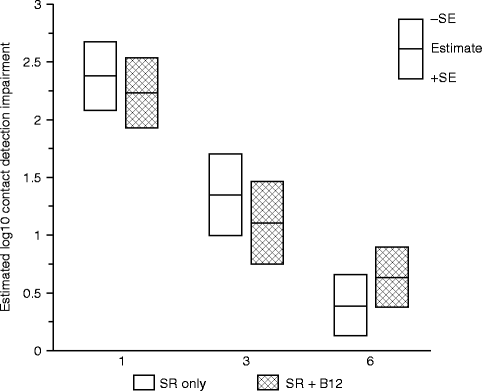

Fig. 18.5
Impairment in contact (touch) detection threshold (CDT) on the chin. Shown are the geometric least-square means of the log10 impairment ratio separately by treatment group controlling for age at surgery, type of surgery, and presence of a genioplasty at 1, 3, and 6 months postsurgery (Data from Phillips et al. [66])
Alternatively, since B12 supplementation was initiated 2–3 weeks before the injury, the earlier recovery in the SR + B12 group may indicate a neuroprotective effect. This would be important clinically if a nerve injury is anticipated to occur.
18.16 Two-Point Perception
Because sensory retraining serves to compensate for the altered sensation experienced by patients in response to mechanical stimulation, we hypothesized that sensory retraining plus vitamin B12 might not improve two-point perception beyond that achieved from sensory retraining alone. Consistent with this hypothesis, there was little or no difference between the SR + B12 and SR-only groups in the adjusted estimates of two-point perception at 1, 3, and 6 months postsurgery. Moreover, the levels of impairment postsurgery were similar to those observed in our previous study of sensory retraining.
18.17 Thermal Sensibility
For all four threshold measures, less impairment was observed for the SR + B12 group than the SR-only group. However, a greater difference was observed for the warm and heat discomfort perception thresholds than for the cool and cold discomfort perception thresholds. Moreover, at 3 and 6 months, subjects on average exhibited hypersensitivity to cold, but less so for those who received vitamin B12 than those who did not. Although a possibility, the difference in instructional protocol (cold at threshold uncomfortable vs. cold at threshold perceived to be burning, pricking, or stinging) prohibits us from suggesting that vitamin B12 may potentially reduce the likelihood or severity of cold allodynia. The large difference between the two groups in the warm perception threshold and heat discomfort perception threshold is consistent with a difference in the functional innervation densities of the unmyelinated afferents that serve these two thermal sensibilities. Even at 6 months postsurgery, subjects who did not receive vitamin B12 required temperatures to be about 4.5 °C hotter than before surgery to experience heat discomfort (Fig. 18.6) [66]. In contrast, subjects who received vitamin B12 required temperatures to be only about 1.5 °C hotter.
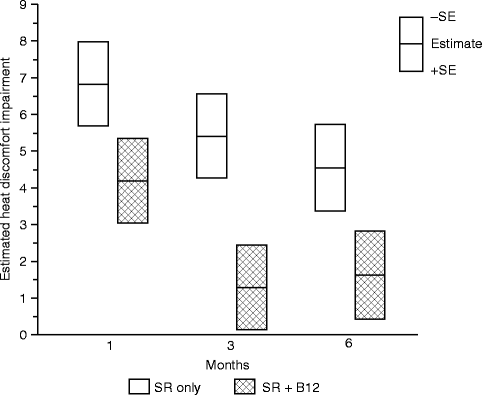

Fig. 18.6
Impairment in the heat discomfort threshold (HPT) on the chin. Shown are the least-square means of the impairment in °C, separately by treatment group controlling for age at surgery, type of surgery, and presence of a genioplasty at 1, 3, and 6 months postsurgery (Data from Phillips et al. [66])
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


