Lipids
Publisher Summary
The term “lipid” covers a miscellaneous collection of compounds having a predominantly hydrocarbon nature. They are consequently insoluble in water and readily soluble in most organic solvents. Apart from solubility considerations, inclusion of a compound in the group usually depends on its content of long chain fatty acids. Lipids are subdivided into simple lipids, compound lipids, and steroids. Lipids are widely distributed throughout both plant and animal kingdoms and are essential constituents of cell membranes; the phospholipids and cholesterol are particularly significant in this respect. Quantitatively, the triglycerides are the most abundant of the lipids and form the chief energy reserve of animals. They also protect the body from heat loss and mechanical injury. In plants, triglycerides occur in quantity only in seeds. The waxes have a protective function. In animals, they keep feathers, skin, and hair soft, pliable, and water repellant, while the cuticle waxes of plants protect them both from dehydration and from invasion by harmful organisms.
1. Simple lipids. These consist of long chain fatty acids which may be either free or combined with an alcohol by an ester linkage. They include the triglycerides (triacylglycerols) and the waxes.
2. Compound lipids which contain additional groupings such as phosphoric acid, sugars, nitrogenous bases or proteins. Included in this group are the phospholipids, glycolipids and lipoproteins.
3. Steroids. Although they do not often contain fatty acids the steroids are frequently classed as lipids on account of their occurrence in natural fats and their solubility characteristics. They include cholesterol and the sex and adrenocortical hormones.
Simple lipids
Naturally occurring fatty acids
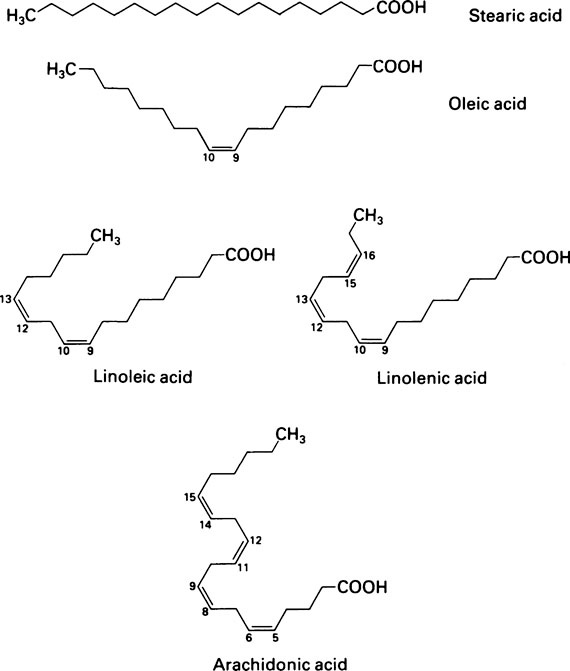
The fatty acids were originally given Greek names indicating their source but their systematic names based on Greek numbers indicate not only the length of the chain but also the degree of saturation. The suffix -anoic indicates that the acid is saturated and -enoic that it is unsaturated. If there are two or three double bonds the suffix becomes -dienoic and -trienoic respectively. The carbon atoms are numbered along the chain, beginning with C-1, the carboxyl group. Alternatively the carbon atom adjacent to the -COOH group is known as the α-carbon atom and the one furthest from the -COOH group as the ω-carbon atom. The position of the double bond may be indicated by inserting the lower of the numbers of the two carbon atoms involved in the bond. Thus the systematic name for oleic acid which has 18 carbon atoms and a double bond between carbons 9 and 10 is 9-octadecenoic acid while that of linoleic acid which contains 18 carbon atoms and double bonds between carbon atoms 9 and 10 and between 12 and 13 is 9,12-octadecadienoic acid (Tables 8.1 and 8.2).
Table 8.1
Naturally occurring straight chain saturated fatty acids
| No. of C atoms | Common name | Systematic name | |
| 2 |  |
Short chain |  |
| 3 | |||
| 4 | |||
| 8 | 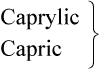 |
Medium chain | 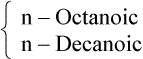 |
| 10 | |||
| 12 |  |
Long chain |  |
| 14 | |||
| 16 | |||
| 18 | |||
| 20 |
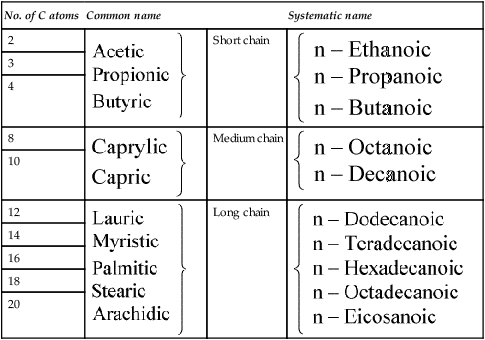
Table 8.2
Some naturally occurring unsaturated fatty acids
| No. of C atoms | No. of double bonds | Common name | Systematic name |
| 16 | 1 | Palmitoleic | cis-9-Hexadecanoic |
| 18 | 1 | Oleic | cis-9-Octadecenoic |
| 18 | 2 | Linoleic* | cis, cis-9,12-Octadecadienoic |
| 18 | 3 | γ-Linolenic* | all-cis-6,9,12-Octadecatrienoic |
| 20 | 4 | Arachidonic* | all-cis-5,8,11,14-Eicosatetraenoic |
Unsaturated fatty acids
Oleic acid which contains a single double bond in the middle of a C18 chain is one of the most abundant fatty acids. Other members of the series are found in smaller amounts as are a number of polyunsaturated fatty acids containing more than one double bond (Table 8.2). These include linoleic acid and arachidonic acid which, although present in relatively small amounts, are physiologically important. They cannot be synthesized in the body and must therefore be supplied in the diet (page 123). Hence they are known as essential fatty acids.
< ?xml:namespace prefix = "mml" />
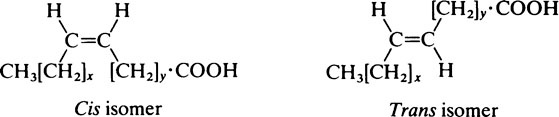
The hydrocarbon chains of saturated fatty acids (and unsaturated fatty acids in which the configuration is all trans) assume a zigzag configuration with the C-C bonds forming a bond angle of 109°; if there is a cis double bond present, the zigzag alters course at this point. Since the naturally occurring unsaturated fatty acids usually have a cis configuration about their double bonds their chains tend to be less compact than those of saturated acids, which can fit into smaller spaces (Figure 8.1). This is a significant consideration in membrane structure (page 194) for, whereas saturated fatty acid chains are flexible, the unsaturated ones are kinked and relatively rigid.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses