(1)
Department of Endodontics, Nova Southeastern University College of Dental Medicine, Fort Lauderdale, FL, USA
Instrumentation of the root canal alone is not sufficient to remove infected necrotic tissues [1]. An irrigating solution is needed to reduce the friction between the instrument and dentin, improve the cutting effectiveness of the files and instruments, dissolve the tissue, cool the file and tooth, wash the debris from the root canal, and be bactericidal in areas of the canal which could not be instrumented [2, 3]. Few irrigating solutions can remove smear layer, so a chelating agent must be used after the irrigating solution to help clean the instrumented root canal surfaces [4]. Through experience, most dentists dilute sodium hypochlorite and use it as an irrigating solution during root canal instrumentation; then they use EDTA or another chelating agent to remove smear layer [5]. The use of sodium hypochlorite as an irrigating solution followed by a rinse of EDTA can produce reliable results [6]. The bactericidal effectiveness of sodium hypochlorite is because it is highly toxic and caustic [7]. If sodium hypochlorite is accidentally spilled on the tissue, it can severely injure a patient [8]. There are procedures in using sodium hypochlorite and chelating agents, which can improve patient safety, and alternative irrigating solutions that may be useful [9].
Irrigating the Infected Root Canal
Hundreds of bacterial species inhabit the mouth [10]. However, because of bacterial interactions, nutrient availability, and low-oxygen potentials in root canals with necrotic pulp, the number of bacterial species present in endodontic infections are restricted [10]. These selective conditions lead to the predominance of facultative and strictly anaerobic microorganisms that survive and multiply, causing infections that stimulate local bone resorption, and are more resistant to endodontic treatment [10]. Among the types of bacteria that infect the root canal, Enterococcus faecalis (E. faecalis) is the one most commonly associated with failed endodontic treatment [11]. In addition to bacteria, the root canal can also be infected by viruses [12] and Candida albicans [13]. The disinfection of root canals through the elimination of microorganisms is an essential step in endodontic treatment (Garcia 3) [14] to help avoid subsequent failure (Garcia 10) [15]. Surface adherence by bacteria to form biofilms is a good example of bacterial adaptation and one that is pertinent to endodontic infections. Increasing information is now available on the existence of biofilm communities on root canal walls (Garcia 28) [16]. Unfortunately, complete root canal disinfection is difficult to accomplish; microorganisms can remain within the apical dentin plug (Garcia 15) [17], within the smear layer (Garcia 8) [18], and within the dentinal tubules (Garcia 18) [19]. To maximize the removal of microorganisms from the root canal, the shaping and mechanical enlargement of a root canal must be accompanied by copious irrigation (Garcia 10,13) [15, 20]. Disinfecting the root canal to reduce the quantity of bacteria, viruses, and fungus to low levels which cannot cause a flare-up is an important requirement of endodontic treatment.
Functions of Irrigating Solutions
The root canal must never be instrumented dry, and an irrigating solution is always needed to reduce the amount of friction between the instrument and dentin surface to prevent binding and sticking [21]. The irrigating solution is needed to increase the amount of cutting that the blades of the hand files and endodontic instruments can perform within time constraints [22]. The irrigating solution must dissolve necrotic and infected tissues within the canal to help clean and disinfect it [23]. Temperature increases, as low as 5 °C, can injure the tissues [24]; the irrigating solution is needed to dissipate the heat generated by instrument friction [25]. The irrigating solution must be able to wash the debris from inside the root canal to help clean it [26]. The irrigating solution must be bactericidal to infected tissues inside the canal which could not be reached by the blades of hand files and endodontic instruments [2, 3].
Types and Dilutions of Irrigating Solutions
The selection of an irrigating solution is important to the outcome of root canal treatment: a solution such as distilled water is entirely inappropriate for root canal irrigation, because although it can remove loose debris, it does not have the chemical ability to disinfect the root canal or to digest the necrotic tissues. Alternatively, many dentists will use undiluted sodium hypochlorite (6–8 % bleach), which has a powerful disinfection and tissue digestion properties but is also highly toxic to any tissues it comes into contact with. To minimize sodium hypochlorite accidents, the irrigating needle should always be placed at least 1-mm short of the working length and fit loosely in the canal. The sodium hypochlorite should also be injected slowly to obtain a gentle flow rate. While injecting the sodium hypochlorite, the needle should be moved up and down the canal to give better irrigation. The irrigation tips should be side venting to help reduce the risk of forcing sodium hypochlorite through the root apex into the periapical tissues. In most cases, the postaccident treatment of patients where sodium hypochlorite has been spilled is palliative care, and observing the patient to ensure the injury does not spread, in addition to prescribing antibiotics and analgesics.
Most dentists will compromise and dilute the sodium hypochlorite with water and use a 3–4 % concentration of sodium hypochlorite for irrigating the root canal [27], while more inexperienced dentists will dilute the sodium hypochlorite with water to a 2–0.5 % concentration [28]. The reason why dentists dilute the sodium hypochlorite is to reduce the amount of injury it can cause to the patient if it gets accidentally spilled out of the root canal [8].Thus, more experienced dentists who are more confident of not spilling the sodium hypochlorite have a tendency to use higher concentrations, while dental students who lack confidence in their skills to avoid spillage will tend to use lower concentrations. In addition to experience and skill, if the root apex is open in an immature tooth, then the sodium hypochlorite must be diluted to approximately 1.25 % with water for root canal irrigation because of the high risk that it can leak through the apical foramen into the periapical tissues [29]. A flow chart for deciding on the concentration of sodium hypochlorite to use is shown in Fig. 7.1.
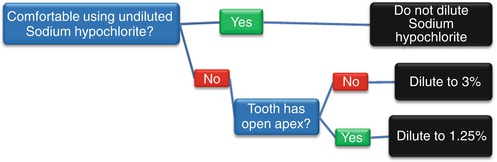

Fig. 7.1
Flow chart for diluting sodium hypochlorite as a root canal irrigating solution
Sodium hypochlorite has been the most widely used root canal irrigating solution for several decades, because it is inexpensive, can quickly dissolve infected necrotic tissues, and is bactericidal [30, 31]. It is very toxic to tissues when undiluted and so accidental spillage is always a concern among dentists [32, 33]. Moreover, sodium hypochlorite by itself cannot completely clean the surfaces of root canals, and it cannot remove the smear layer created by instrumentation [34]. A few dentists will use alternative root canal irrigating solutions to sodium hypochlorite, and these include chlorhexidine gluconate, an activated water called Aquatine Endodontic Cleanser, or a natural fruit juice extract such as Morinda citrifolia.
A 2 % solution of chlorhexidine gluconate (CHX) has good bactericidal properties to disinfect the root canal [35, 36]. CHX is a bis-bis-guanide with amphiphatic and antiseptic properties [37]. CHX is biocompatible to tissues [36] so it is less harmful when spilled. However, the use of CHX as an endodontic irrigant is generally restricted because it cannot dissolve infected necrotic tissues. CHX can also discolor the teeth [38], and if it is spilled a patient might experience side effects such as loss of taste, burning sensation of the oral mucosa, subjective dryness of the oral cavity, and discoloration of the tongue [39], and it is also less effective to dissolve necrotic infected tissues. Generally, chlorhexidine gluconate is not a good alternative irrigating solution to sodium hypochlorite, because even at full strength, its ability to clean the root canal surfaces is inferior to sodium hypochlorite (Yamashita et al. 1993) [40].
In August 2006, the US Food and Drug Administration approved Sterilox Dental’s Aquatine Endodontic Cleanser (Aquatine EC, Sterilox Puricore, Malvern, PA, USA) for use as an endodontic irrigating solution. The active component in Aquatine EC is hypochlorous acid (HOCl) [41]. HOCl is produced by the human body’s immune cells, through a chain of aerobic reactions called the oxidative burst pathway, to kill invading pathogens and to fight infection (Garcia 6) [42]. Aquatine EC is produced by electrochemically charging a low-concentration salt solution using an element reactor. HOCl is commonly used for hospital disinfection and sterilization and in the treatment of chronic wounds (Garcia 25) [43]. In dentistry, it is commonly used to disinfect water lines by removing biofilms (Garcia 7,12) [44]. HOCl is biocompatible to the tissues and antimicrobial against a broad range of microorganisms (Garcia 12) [45]. Two in vitro studies have demonstrated that freshly made HOCI solution can be effective as an endodontic irrigating solution (Garcia 27) [46]. However, there are no long-term clinical trials which have demonstrated that a HOCI solution is as effective as an irrigating solution as sodium hypochlorite.
Some patients and dentists are searching for natural irrigating solutions among plant extracts that have some bactericidal properties. Few plant extracts are suitable as an endodontic irrigating solution because they contain natural sugars which could feed bacteria infecting a root canal. The antimicrobial effects of natural fruit juices and plant extracts on E. faecalis and other endodontic pathogens have generally not been evaluated, except for the Arctium lappa plant extract, which was effective at disinfecting ex vivo root canals (6) [47], and fruit juice from the exotic Morinda citrifolia or noni plant (Garcia article + new article) [48, 49]. Morinda citrifolia juice (MCJ) has a broad range of therapeutic effects, including antibacterial, antiviral, antifungal, antitumor, anthelmintic, analgesic, hypotensive, anti-inflammatory, and immune-enhancing effects (Garcia 1–3) [14, 34, 50]. MCJ contains the antibacterial compounds L-asperuloside and alizarin (Garcia 4) [51]. Acetone extracts from MCJ also demonstrated some antimicrobial activity (5) [52].
While some fruit juices and plant extracts, especially from plant roots, may be appealing to the growing patient base who wants to have treatment only using natural remedies, these compounds are expensive, and there is no long-term clinical evidence that root canal irrigation with natural irrigating solution is beneficial.
Functions of Chelating Agents
A severe limitation of sodium hypochlorite and most other irrigating solutions is that they are unable to dissolve the instrumentation debris on cut dentin surfaces, called smear layer [53]. The smear layer is a 1–5-μm-thick layer of cut debris created on the surface of instrumented dentin, composed of dentin, odontoblastic processes, nonspecific inorganic contaminants, and microorganisms (Garcia 5) [54]. The smear layer can harbor infected necrotic tissue, bacteria, bacterial products, and root canal remnants [55]. The presence of smear layer can prevent the adequate sealing of the root canal with sealers, thereby creating pathways for bacterial leakage (Garcia 23) [6] which may lead to a failure of endodontic treatment. The presence of smear layer on the instrumented root canal surface and its removal with a common chelating agent are shown in Fig. 7.2.
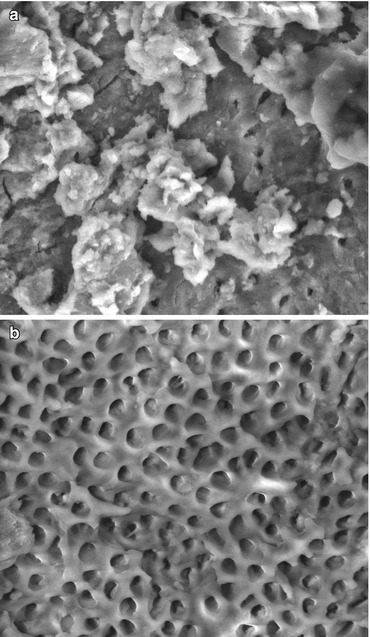

Fig. 7.2
Scanning electron micrographs of smear layer and its removal from the root canal surface. (a) Smear layer covering the root canal surface after instrumentation and irrigating with sodium hypochlorite without a chelating agent. (b) Open dentinal tubules of an instrumented root canal surface showing that the smear has been removed by irrigating with sodium hypochlorite and rinsing with 17 % EDTA
The removal of smear layer from the instrumented root canal walls is controversial [56]. Its removal provides better sealing of the endodontic filling material to dentin and will avoid the leakage of microorganisms into oral tissues (Sen et al. 1995) [57]. The infiltration of microorganisms into oral tissues must be prevented because these often cause complications leading to treatment failure. Unfortunately, smear layer is difficult to entirely remove from instrumented root canals, particularly in the constricted apical region [4].
The most widely used chelating agent inside the root canal is 17 % ethylenediaminetetraacetic acid (EDTA) [58]. It has good chelating properties to remove smear layer and clean the surface of the root canals [59]. Testing and clinical evidence has shown that 17 % EDTA needs to be placed inside the root canal for 1 min to effectively dissolve organic components and smear layer [60]. If the EDTA is placed within the root canal for less than 1 min, the smear layer will not be optimally removed; if the EDTA is placed within the root canal for more than 1 min, there is a risk that its chelating effect will weaken tooth structure. A solution of 17 % EDTA is a very reliable endodontic chelating agent when used fresh and at room temperature, but its chelating effects are time sensitive [61], and it should never be kept within the root canal for more than 1 min. The EDTA then needs to be suctioned, dried with paper points, and/or rinsed with sodium hypochlorite to ensure it has been completely removed from the root canal after use [62].
The BioPure MTAD Antibacterial Root Canal Cleanser (MTAD) is an alternative chelating agent to 17 % EDTA, and it is one of the newest endodontic chelating agents available on the market [63]. MTAD has the least published data available, but it can clean the root canals, digest the tissues, and has bactericidal properties that are equal or better than full-strength sodium hypochlorite [64]. Some other in vitro studies claim that 6 and 1 % solutions of sodium hypochlorite were more effective than BioPure MTAD to disinfect E. faecalis biofilms from the root canals [65]. MTAD contains a broad-spectrum antibiotic called doxycycline, in addition to citric acid and a detergent [66]. The sustained antimicrobial activity of MTAD is superior to CHX (AAE30) [67]. MTAD is biocompatible and can enhance the bond strength of sealers to the tooth structure (AAE14) [68]. The effectiveness of MTAD to remove the smear layer is enhanced when a 1.3 % concentration of sodium hypochlorite is used as an intracanal irrigant. One milliliter of MTAD is placed within the root canal for 5 min, and it is rinsed with an additional 4 ml of MTAD as the final rinse (AAE33) [69]. The main disadvantage of MTAD is that it is a more expensive alternative to sodium hypochlorite for irrigating the root canals.
The Qmix 2in1 Endodontic Cleanser (Qmix) is an alternative chelating agent to 17 % EDTA or MTAD. Unlike MTAD, the Qmix does not contain any antibiotics. Qmix contains a mixture of a bisbiguanide antimicrobial agent, a polyaminocarboxylic acid calcium-chelating agent, and a surfactant. Qmix has been found to be effective against bacterial biofilms [70]. Qmix is as effective as 17 % EDTA, when it is placed in the root canals for between 60 and 90 s after irrigation with sodium hypochlorite [71].
A comparison of the removal of the smear layer from ex vivo root canals which were instrumented and irrigated with sodium hypochlorite, followed by the chelating agents, 17 % EDTA, MTAD, or Qmix CHX, is shown in Fig. 7.3.
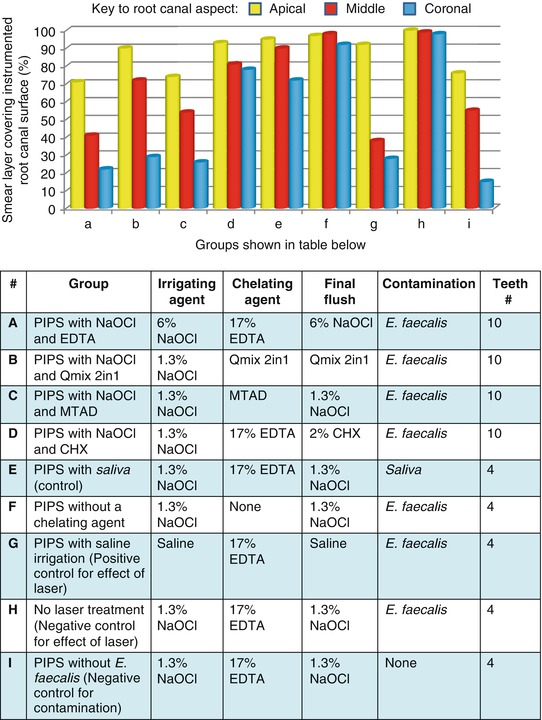

Fig. 7.3
A comparison of the effectiveness of irrigating solutions and chelating agents to remove smear layer from the instrumented canals of ex vivo teeth when activated with photon-induced photoacoustic streaming (PIPS)
Activation of Irrigating Solution and Chelating Agents
The process of canal preparation with files, instruments, and irrigating solutions is usually sufficient to remove most of the necrotic and infected tissues. Some recent articles suggest that the ultrasonic activation of irrigating solutions [72] the use of highs-speed vacuum; the EndoVac system [73] and that a laser using photon-induced photoacoustic streaming (PIPS) can improve the debridement of root canals [74]. The effect of cleaning and shaping the root canals followed by PIPS is shown in Fig. 7.4.
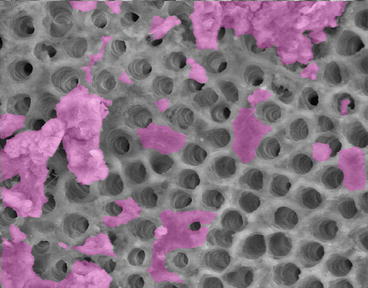

Fig. 7.4
Scanning electron micrograph of an instrumented root canal surface with open dentinal tubules where the smear layer and debris have been removed. The purple indicates where there is smear layer or debris still attached to the root canal surface
Summary
The root canals should be irrigated with sodium hypochlorite during instrumentation. Undiluted sodium hypochlorite is the most effective concentration, but it may be diluted according to the experience of the dentist or because a tooth is immature and has an open apex. It is not enough to use sodium hypochlorite to clean the root canals following instrumentation. A chelating agent is also needed; most dentists will use EDTA, although there are other effective products such as MTAD and Qmix available. The effectiveness of the irrigation solution and chelating agent to remove smear layer and to clean the canals can be improved by activating the solutions with ultrasonics, by high-speed suction such as the EndoVac system, or by a laser system such as PIPS.
Quiz for the Topics Covered in Chapter 7
1.
Hundreds of bacterial species inhabit the mouth, because of bacterial interactions, nutrient availability, and low-oxygen potentials in root canals with necrotic pulp; the number of bacterial species present in endodontic infections is restricted.
(a)
False
(b)
True
2.
These selective root canal environmental conditions lead to the predominance of facultative and strictly anaerobic microorganisms that survive and multiply, causing infections that stimulate local bone resorption, and are more resistant to endodontic treatment.
(a)
False
(b)
True
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


