Introduction
The purpose of this study was to examine the effect of a new cryopreservation method with a magnetic field on periodontal regeneration in vitro and in vivo.
Methods
Human periodontal ligament cells were frozen in 10% dimethyl sulfoxide by using a programmed freezer with a magnetic field. Cells were cryopreserved for 3 days at −150°C. Immediately after thawing, collagen type I and alkaline phosphatase gene expression were determined by real-time polymerase chain reaction. Incisors were extracted from 15-week-old Wistar rats and cryopreserved or dried for 3 days. Then the incisors were replanted into the same sockets. Ninety days after transplantation, they were observed under light microscopy.
Results
There was no difference in the messenger RNA expression of collagen type I between the cryopreserved and the control groups. The expression of alkaline phosphatase messenger RNA in the cryopreserved group was slightly decreased compared with the control group. There was no progressive root resorption in the teeth that were replanted immediately (control group) or cryopreserved. However, there was widespread root resorption and ankylosis in the dried teeth.
Conclusions
These results show that a magnetic field programmed freezer can be successfully used for cryopreservation of teeth.
Tooth autotransplantation is a useful procedure for recovering occlusal function by replacement of missing teeth. Wisdom teeth, impacted teeth, and unnecessary teeth that are extracted for orthodontic treatment are mainly used as donor teeth. Tooth autotransplantation restores the function of lost teeth and avoids the loss of intact adjacent teeth, unlike conventional prosthetic treatment. Autotransplanted teeth can obtain periodontal sensitivity and can be moved orthodontically. Moreover, the autotransplantation of a healthy tooth can replace the destroyed alveolar bone through the differentiation capacity of periodontal ligament (PDL) cells. The survival rate of immediately autotransplanted teeth exceeds 95% when root formation is not complete. However, sometimes patients do not have a donor tooth available because it was previously extracted. To solve this problem, tooth cryopreservation systems have been developed. Many clinical reports and animal experiments show the efficacy of tooth cryopreservation. However, in some cases, the transplantation of a cryopreserved tooth can result in tooth-bone ankylosis or root resorption because of PDL cell damage induced by ice crystal formation inside the cells as well as mechanical stresses caused by extracellular ice formation. Two approaches, vitrification and slow freezing, have been tried to achieve cryopreservation without cell damage. Vitrification is a process by which the cells freeze quickly before ice crystals can form. Although this approach is efficient, it requires a high concentration of cryoprotectants that are usually toxic to most cells. On the other hand, conventional slow freezing requires a low, relatively nontoxic, concentration of cryoprotectants; however, it is always associated with cell injury from ice formation and prolonged exposure to cryoprotectants. Therefore, it is necessary to develop a new technology that can prevent ice formation without a high concentration of cryoprotectants.
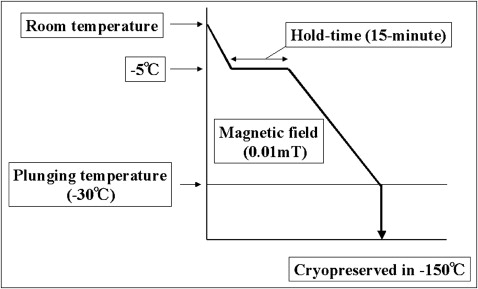
Recently, we developed a special freezer called the “Cells Alive System” (ABI Corporation, Chiba, Japan), which uses a magnetic field. Cells contain a cluster of water molecules. When cells freeze, the cluster grows and injures the cell membrane. However, a magnetic field can prevent the cluster from growing by causing it to vibrate. Our previous findings showed that a 0.01-mT magnetic field, a 15-minute hold time, and a plunging temperature of −30°C led to the greatest survival rate for PDL cells. The PDL cells of teeth that were cryopreserved with a magnetic field could proliferate as much as the PDL cells of fresh teeth in an organ culture. However, autotransplantation of cryopreserved teeth by the Cells Alive System and the characterization of their PDL cells have not yet been performed.
In this study, we examined gene expression in human PDL cells after cryopreservation for 3 days. We also investigated periodontal healing after replantation of a rat incisor that was cryopreserved by the Cells Alive System.
Material and methods
Human PDL cells were isolated from first premolars that were extracted during orthodontic treatment (10 patients; mean age, 18 years 8 months) as reported earlier. This study was approved by the ethics committee of Hiroshima University, and informed consent was obtained before tooth extraction. Cells were cultured in α-modified eagle’s medium (Sigma, St Louis, Mo) containing 10% fetal calf serum (Biological Industries, Kibbutz Beit-Haemek, Israel), 32 U per milliliter of penicillin G (Meiji Seika, Tokyo, Japan), 250 μg per milliliter of amphotericin B (Nacalai Tesque, Kyoto, Japan), and 60 μg per milliliter of kanamycin (Meiji Seika) at 37°C in a humidified atmosphere of 5% carbon dioxide. When the cells became confluent, the monolayers were treated with 0.1% trypsin/EDTA and replated under the same conditions. Cells from passages 3 to 4 were used for all experiments according to a previous study by Min et al.
A total of 105 cells were placed in a tube with 1 mL of preservation media (Bambanker 2, Lymphotec, Tokyo, Japan) that contained 10% dimethyl sulfoxide. The cells were frozen by a Cells Alive System programmed freezer containing a magnetic field (ABI Corporation). The Cells Alive System freezer was programmed to freeze cells at −0.5°C per minute with a 0.01-mT magnetic field, a 15-minute hold time at −5°C, and a plunging temperature of −30°C, according to our previous study ( Fig 1 ). Cells were cryopreserved for 3 days at −150°C and then thawed in a 37°C water bath. The cryopreserved PDL cells were cultured and grown in the same way as for the noncryopreserved cells (control group).
Total RNA was isolated from the cell cultures by using a Quickprep Total RNA extraction kit (Amersham Biosciences, Tokyo, Japan). Single-stranded complementary DNA was synthesized from 1 μg of total RNA by using an Oligo(dT) 2 0 primer (Toyobo, Osaka, Japan) and a Rever Tra Ace-α first-strand complementary DNA synthesis kit (Toyobo).
The following primers were used: collagen type I, 5′-CCGTGGCAACTCTATCTTTGG-3′ (sense) and 5′-GCCATACAGGATGGCAGTGA-3′ (antisense); alkaline phosphatase, 5′-CCGTGGCAACTCTATCTTTGG-3′ (sense) and 5′-GCCATACAGGATGGCAGTGA-3′ (antisense); and G3PDH (Rever Tra Ace-α first-strand complementary DNA synthesis kit, as the control, 5′-ACCACAGTCCATGCCATCAC-3′ (sense) and 5′-TCCACCACCCTGTTGCTGTA-3′ (antisense).
We measured the collagen type I and alkaline phosphatase gene expression of PDL cells from immediately extracted teeth (control group) and the gene expression of cryopreserved PDL cells in a Cells Alive System freezer for 3 days. Real-time polymerase chain reaction was performed with SYBR Green I assay and ABI Prism 7700 sequence detection system (Amersham Biosystems) with 1 μL of sample complementary DNA by using 3-stage program parameters as follows: 2 minutes at 50°C, 10 minutes at 95°C, and then 40 cycles at 45 seconds at 94°C, 45 seconds at 55°C, and 45 seconds at 72°C. Quantitative results from real-time fluorescence polymerase chain reaction were assessed by a cycle threshold value, which identifies a cycle when the fluorescence of a sample becomes significantly different from the baseline signal. Relative quantification of the collagen type I and alkaline phosphatase signals was normalized and expressed relative to the G3PDH signals. Polymerase chain reactions for each sample were repeated 3 times with 1 sample for both the target gene and the control.
Fifteen-week-old male Wistar rats (n = 15) were anesthetized with pentobarbital (10 mL/kg), and their maxillary right incisors were extracted. In the control group (n = 5), the teeth were replanted into the same sockets immediately after extraction. In the cryopreservation group (n = 5), the incisors were put into media that contained 10% dimethyl sulfoxide and cryopreserved for 3 days before being replanted into the same sockets of the same rats. The incisors of the remaining group were dried for 3 days and then replanted into the same sockets of the same rats as a negative control. The animals were able to eat without difficulty because we took out only the maxillary right incisor for just 3 days. So, the effect of the surgical procedure was closely observed. In the experiment period, there was no statistical difference in the body weight among these groups.
Ninety days after replantation, the rats were killed under general anesthesia with sodium pentobarbital. The rats were fixed in 4% paraformaldehyde and rinsed in distilled water. Then, the specimens were decalcified in 14% EDTA (pH 7.4) for 30 days and embedded in paraffin. The premaxillary bones, including the maxillary incisors, were cut into frontal sections 7 μm thick. After deparaffinization with xylene and dehydration with ethanol, the sections were stained with hematoxylin and eosin. Then the periodontal healing of the transplanted teeth was observed microscopically. All experiments were performed in accordance with protocols approved by the committee of research facilities for laboratory animal science at Hiroshima University School of Medicine.
Statistical analysis
Differences between the mean values of the real-time polymerase chain reaction results from the control and cryopreserved groups were examined with the Student t test with Statview software (Abacus Concepts, Berkeley, Calif). A confidence level of P <0.05 was considered statistically significant.
Results
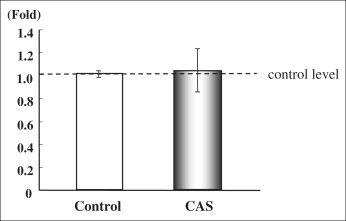
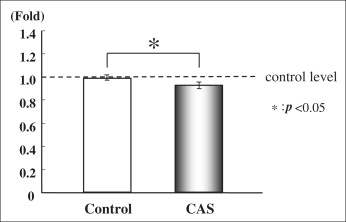
We measured the collagen type I and alkaline phosphatase gene expression of cultured PDL cells from the control group (noncryopreserved cells) and the cryopreserved group (cryopreserved for 3 days before polymerase chain reaction). There was no significant difference between the 2 groups in the expression of collagen type I messenger RNA (mRNA) ( Fig 2 ). The level of alkaline phosphatase mRNA in the cryopreserved group was 90% of the control group level, which was a statistically significant difference ( P <0.05, Fig 3 ).
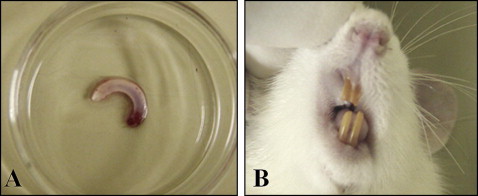
Photographs of an extracted rat incisor and replanted tooth after 3 months are shown in Figure 4 , A and B . Replanted incisors after the 3 months of recovery showed normal healing. Histologic examinations were performed 90 days after replantation. In the control group, periodontal tissues consisting of acellular cementum, PDL, and alveolar bone were similar to those of a normal periodontium. The PDL remained on the cementum surface, and there were some epithelial rests of Malassez. Inflammatory root resorption and replacement resorption were rarely observed ( Fig 5 , A ). In the cryopreserved group, the periodontal tissues were similar to those of the immediately replanted control group, and there was no difference in the amount of PDL space between these 2 groups. Alveolar bone was apart from the root surface with healthy PDL, and root resorption was rarely observed. Epithelial rests of Malassez and many blood vessels were detected in the PDL. There was no effect of the Cells Alive System cryopreservation on the mechanical properties of the tooth, such as root fracture ( Fig 5 , B ). The PDL in the dried group had withered, and there were many areas with direct contact between alveolar bone and root. Progressive replacement root resorption was observed in the dried group. Also, inflammatory cells had infiltrated the nearby alveolar bone ( Fig 5 , C ). There were some atrophied PDL cells in the control group ( Fig 6 , A ), although there were more atrophied and small cells in the cryopreserved group ( Fig 6 , B ). In the control and cryopreserved groups, replanted teeth showed continuous eruption, and there were no differences in the shape and the color. However, in the dried group, eruption stopped because of dental ankylosis.