Fig. 20.1
Sensory response to nerve injury (Adapted from Zuniga and Essick [63]; with permission)
Table 20.1
Common functional impairments for the injured inferior alveolar and lingual nerve
|
Difficulty masticating food bolus
|
|
Drooling
|
|
Difficulty with speech
|
|
Decreased taste sensation
|
|
Difficulty maintaining oral hygiene
|
|
Biting of lip, cheek, tongue
|
|
Decreased sense of self-worth
|
Patients should also be queried as to whether the pain or numbness is progressive in nature or has improved or subsided since the injury. In those instances where a patient has received previous surgical or medical care, the response to such care is important to determine so that an accurate assessment of the type and efficacy of future treatment can be made.
20.2 Physical Examination and Neurosensory Testing
The clinical neurosensory examination is the most important tool in deciding the most appropriate type of treatment that would potentially benefit the patient. As noted above, a detailed history of the injury along with current complaints followed by subjective and objective testing will organize patients into groups in which treatment decisions can be made. The patient history will aid the clinician in deciding whether the patient has decreased overall sensation or pain (Fig. 20.1). Once this determination is made, further testing is performed according to the appropriate algorithm.
Patients with decreased sensation are those who experience a loss of sensation without pain. These patients either have a diminished response to a painful or non-painful stimulus (paresthesia), or are completely without sensation (anesthesia). In the anesthesia scenario, a determination should then be made to establish if a given patient has a mild, moderate, or severe functional impairment. Common functional impairments for the injured IAN and LN are outlined in Table 20.1 [43, 63].
Patients with significant pain after initial questioning follow the other branch of the algorithm in Fig. 20.1. It must first be determined whether the pain is stimulus induced or spontaneous in nature. Stimulus-induced pain is characterized as hyperalgesia (an exaggerated response to sharp pain, e.g., pin prick), allodynia (painful sensation to a stimulus that normally does not cause pain, e.g., shaving, kissing, or brushing teeth), or hyperpathia (a delayed or prolonged response to a normally painful stimulus). On the other hand, spontaneous pain can be either intermittent or constant. Intermittent pain is unpredictable and must not be confused with a delayed response to a hyperpathic stimulus. Constant pain occurs within the deep or superficial tissues and can be a chronic, dull, throbbing ache with or without lancinating electric shock-like sensations [43, 61].
After determining which aspect of the algorithm to follow for a particular patient (Fig. 20.1), a specific physical exam can be tailored for the individual patient to confirm a diagnosis and develop a treatment plan. A complete head and neck examination is followed by a focused inspection of the injured area. The degree and location of injury may be evident by disruption of mucosa, erythema, ulceration, hyperkeratosis, texture changes, or signs of self-induced trauma. Palpation of a trigger response may elicit abnormal sensations at or distal to the injured site (Tinel’s sign). Evaluation of the contralateral or “normal” side is used as a control [61]. The method of neurosensory testing performed is based upon whether the patient has pain or decreased sensation, established during the patient interview. If the patient is without pain, sensory testing proceeds under the “decreased sensation” algorithm. Whereas if the patient has pain, sensory testing proceeds in a different manner [43]. Neurosensory testing for the decreased sensation patient is divided into three levels (Fig. 20.2). Level A testing evaluates for tactile brush stroke direction and two-point discrimination. Tactile brush stroke directional discrimination is assessed by using a soft brush (0.75 × 0.5 cm) stroked in four directions along the test site. This test is repeated and compared to the control side. Two-point discrimination testing measures spatial acuity. The stimulating device must provide two points of skin contact and is readily adjustable. A two-point discrimination of greater than 6.5 mm is a reliable indicator of sensory impairment in the majority of the population. The stimulus should have blunt tips and be applied perpendicular to the skin surface, and pressure is applied to not cause discomfort, blanching, or vibration of the tissue. The stimulus should last 2 s, and the patient is asked to report “one” or “two” with each stimulus application. Two-point distances should be increased and decreased from this measurement and compared to the control side. If the patient consistently discriminates tactile direction correctly and the two-point discrimination exam is ≤6.5 mm, the impairment is extremely subtle and sensory testing may cease. However, if level A testing is abnormal in the “decreased sensation” patient, proceed to level B testing [17]. Level B testing estimates the level of contact detection, using a set of Semmes-Weinstein pressure aesthesiometers (also called von Frey’s hair fibers). The test or injured site threshold is deemed abnormal if it is 250 % less sensitive compared with the control value or greater than two standard deviations above the published normative mean values [17]. Only a few axons require regeneration in order to achieve a normal result to testing. Therefore, patients with test site sensitivity comparable to the control site are designated as being mildly impaired, and there is no need to continue testing. A test site sensitivity less than that of the control or published normative values is considered as having a moderately impaired response and will proceed to level C testing. Level C testing evaluates the test area’s response to noxious stimuli (mechanical and thermal). Mechanical pain can be induced by pinprick; however, this type of testing may cause tissue damage (bleeding) and significant pain on the control side. The responses are subjective and difficult to quantify. An algometer (spring-loaded sharp probe) can be used as opposed to traditional pinprick to quantify the force needed to generate pain in these patients and compared with the control side. Thermal testing is the final level C neurosensory test. Hot (>40 °C) and cold (<20 °C) metal probes are utilized. A probe is warmed or cooled using beakers of hot/cold saline or any other method of temperature regulation and placed over the test and control side for thermal evaluation. A heated stimulus of 50 °C against normal tissue will evoke an intolerable pain response without causing tissue damage. An abnormal thermal pain response is one that requires a heated stimulus that is significantly higher on the test site compared with the control. Patients with a significantly high threshold for level C testing are considered severely impaired, whereas those with a relatively normal response to level C testing will maintain the level B diagnosis of moderately impaired sensation [17].
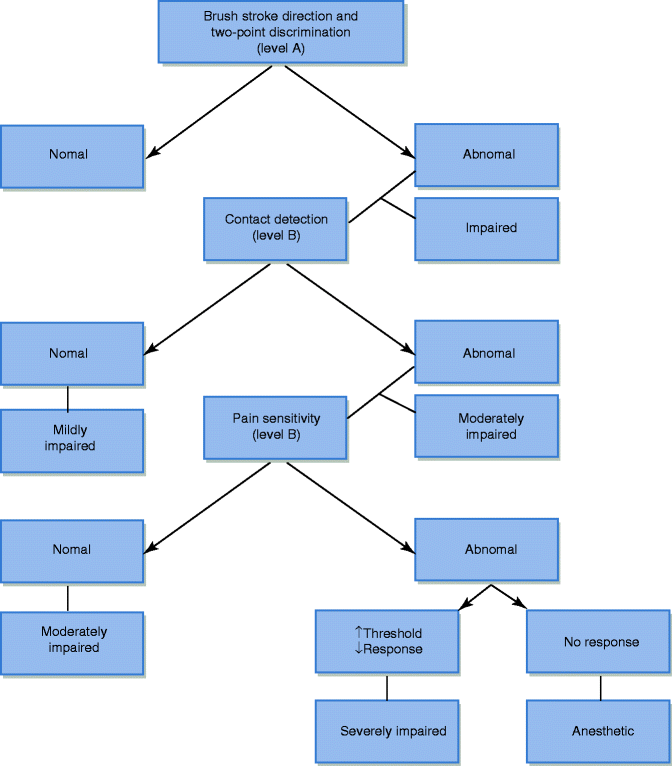

Fig. 20.2
Clinical neurosensory testing
Neurosensory testing for the “painful altered sensation” patient, in contrast to the “decreased sensation” patient, involves all three levels of testing (A, B, and C) for all patients. Level A testing involves an innocuous mechanical stimulus (brush stroke), which may potentially elicit a painful response known as allodynia. A moving brush stroke over the involved area will evoke a painful response over the distribution of the damaged nerve. The clinician should report frequency, duration, and intensity when discomfort is felt by the moving stimulus. Level B testing evaluates for possible hyperpathia in pain patients. These patients exhibit an explosive, radiating, poorly localized pain with a delayed occurrence that may last for an extended period of time. This exam utilizes a suprathreshold von Frey fiber that is repeatedly probed over the test site. Roughly ten repeated tactile pulses are delivered followed by a 1-min rest period to evaluate for delayed pain. Pain frequency, duration, and intensity are recorded by the clinician. Level C testing in the pain patient involves the use of mechanical and thermal noxious stimuli with hot and cold metal probes to elicit a painful response. After level A, B, and C neurosensory testing, the pain patient will undergo diagnostic nerve blocks to evaluate a peripheral versus central etiology of the pain and to assess the contribution of sympathetic output to the maintenance of the pain [38].
A final neurosensory examination performed for both the decreased sensation and pain patient is a taste test challenge. This is performed for lingual nerve damage patients only. Sugar and salt-water beakers are set up without the patient’s knowledge. A cotton tip applicator is dipped into the sugar water and applied over the mucosa of the injured lingual nerve distribution. The patient is questioned if there is any specific taste they are experiencing during the test. After their response, the control side is tested in a similar manner. They must then rinse with water and the examination is repeated using salt water. Responses to these tests are recorded as part of an objective functional impairment.
20.3 Imaging
The history and physical examination of nerve injury patients are the most important part of developing a working diagnosis and making treatment decisions. However, there are some advantages of imaging techniques in some patients. The panoramic radiograph offers limited information due to the two-dimensional nature of the study, but the presence of a foreign body in the region of the LN or IAN may be identified, thus making it a useful screening tool. Rotary instrumentation, remnants of root tips, implant perforation, or root canal filling material may be seen in this study and can aid in localizing the site of injury. The use of computed tomography is helpful in delineating the anatomic relationship between the IAN and the tooth structures. This has facilitated the preoperative risk assessment for IAN injury [26, 56]. Computed tomography (CT) or cone-beam CT (CBCT) imaging may localize a foreign body in three dimensions or illustrate a violation of cortical outline of the inferior alveolar canal or lingual cortical plate. In this regard it can be helpful in planning surgical treatment. However, this modality of imaging provides limited information about the condition of an injured nerve.
Magnetic resonance imaging has been used in various anatomic locations to assess the integrity of large diameter nerves and characterize pathology [6, 8, 9, 30, 40, 46, 49, 58, 60]. High-resolution MRI may offer some helpful information with regard to the condition of the injured LN. A change in nerve diameter may be appreciated due to Wallerian degeneration of the nerve distal to the site of injury, or an acute change in nerve position or shape due to retraction or neuroma formation may be visualized [44]. It is important to note that these imaging modalities can and should only be used as a supplement to a thorough history and clinical neurosensory examination.
20.4 Surgical Treatment Guidelines
The decision to proceed with microneurosurgical treatment is established on an individual basis and depends upon the specific presentation and clinical course for each patient. Surgical repair should be considered when the disability is of concern to the patient and there is clinical evidence of moderate, severe or complete sensory impairment, dystrophic ageusia, or neuropathic pain of peripheral origin. As the clinician follows the serial neurosensory exam over time, careful attention is directed at the extent and character of sensory recovery (Figs. 20.3 and 20.4). The Medical Research Council Scale (MRC), a well-defined guideline for assessing sensory function, was established by Mackinnon and Dellon [39] for extremity injuries (Table 20.2). This has been adapted by others to grade sensory recovery in the domain of the trigeminal nerve [11, 42]. Based upon the response to several neurosensory measurements, a score is assigned which can range from S0 (no recovery) to S4 (complete recovery). If there is clinical evidence of spontaneous restoration of useful protective sensation (MRC score ≥3), then surgical intervention is usually not indicated since both endpoints are similar.
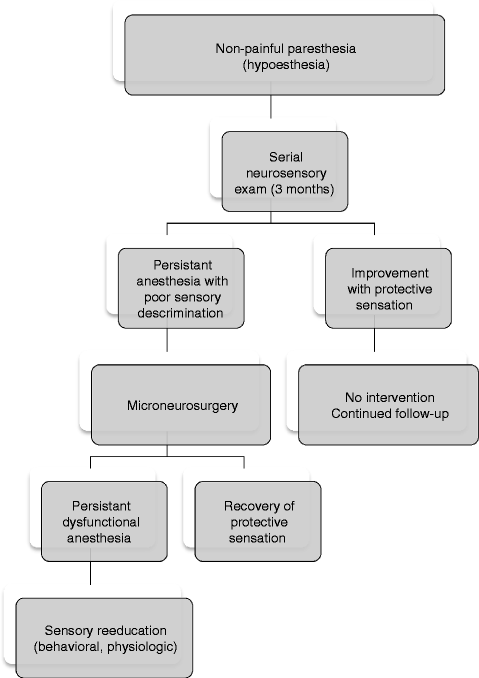
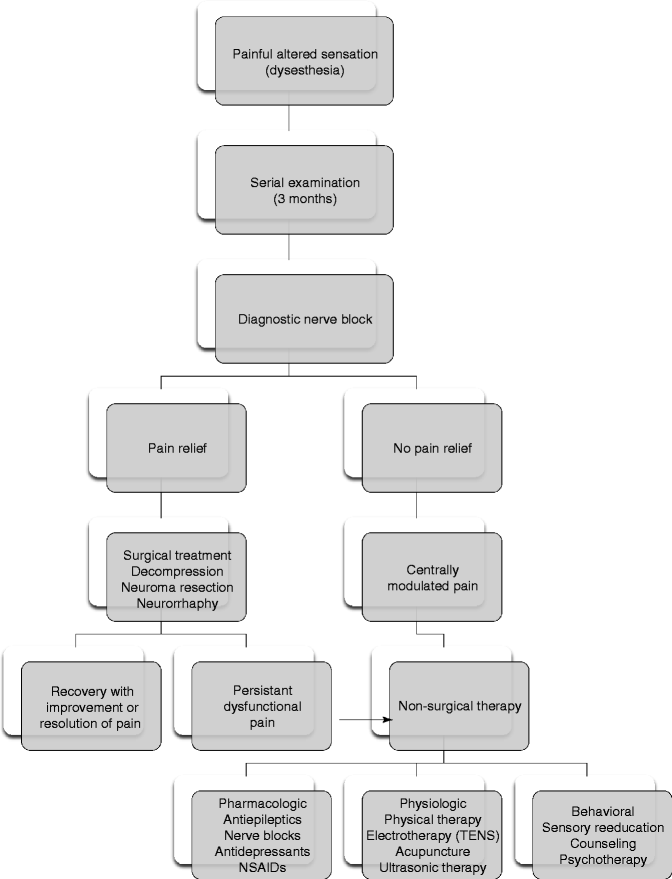

Fig. 20.3
Evaluation and management guidelines for patients with decreased sensation

Fig. 20.4
Evaluation and management guidelines for patients with painful altered sensation
Table 20.2
Medical Research Council Scale (MRCS) of neurosensory recovery
|
Score
|
Description
|
|---|---|
|
S0
|
No sensation
|
|
S1
|
Deep cutaneous pain in autonomous zone
|
|
S2
|
Some superficial pain and touch
|
|
S2+
|
Superficial pain and touch plus hyperesthesia
|
|
S3
|
Superficial pain and touch without hyperesthesia; static two-point discrimination >15 mm
|
|
S3+
|
Same as S3 with good stimulus localization and static two-point discrimination of 7–15 mm
|
|
S4
|
Same as S3 and static two-point discrimination of 6 mm
|
The most important variable to consider is the amount of time that has elapsed since the injury. Since the primary objective for surgical intervention is to reestablish neural continuity of the proximal nerve segment with the distal end organ (lip or tongue), the integrity of the nerve section distal to the site of the injury is pivotal to a successful postsurgical outcome. Following a disconnect from the central nervous system and cell body, the distal nerve segment will undergo atrophy and degeneration (Wallerian degeneration) if it is not exposed to the neurotrophic influences of the proximal segment. As the degeneration continues, the endoneural tubules in the distal segment are replaced with scar tissue that effectively eliminates the potential for axonal repopulation into the distal nerve segment [53, 61]. It has been estimated that within 1 year from injury a significant component of the distal nerve will become atrophied and surgically unrepairable [55, 61]. This has also been substantiated from outcomes and clinical findings during repair attempts in patients with nerve injuries that exceeded 12 months [41]. Despite sporadic reports of recovery several years following a sensory nerve injury [21], most clinical studies have demonstrated poor neurosensory recovery beyond 6 months from the time of injury [5, 13, 31]. In Donoff’s study of 44 lingual and inferior alveolar nerve injuries, they reported a positive correlation with improved sensation and microsurgical repair within 6 months from the injury [14]. Likewise, in Bagheri’s et al. [3] retrospective review of 222 lingual nerve injuries, patients who were repaired more than 9 months following the injury were more likely to experience poor sensory improvement. A logistic regression analysis of their data demonstrated that the odds of improvement decreased by 5.8 % for each month of delay beyond the injury date. For those patients who were repaired within 9 months of the injury, 65 % achieved “complete” return of sensation and 25 % achieved “useful” sensory function as defined by the Medical Research Council Scale (Table 20.2).
In this same study, a relationship was also reported between sensory outcome and age where patients 45 years of age or older were statistically less likely to have a positive outcome [3]. In their analysis, the chance of a recovery decreased by 5.5 % for each year of age beyond 45. In other studies, a similar relationship to age and postsurgical sensory recovery has not been reported [54, 57].
The character of the altered sensation must also be considered prior to proceeding with surgical treatment. The primary treatment objective for patients that present with painful neuropathies is to eliminate or significantly reduce their level of pain (Fig. 20.4). In a multicenter, retrospective study of 521 patients that received surgical treatment, the success rate for patients with hyperesthetic neuropathies was significantly worse than those who presented with non-painful altered sensation [34]. In a study by Gregg [23] the outcome of surgical therapy varied and was dependent upon the nature of the dysesthesia. In this report, the level of pain reduction following microsurgical repair was poor for patients who presented with anesthesia dolorosa (14.6 %) and sympathetic-mediated pain (20.7 %), while those patients with hyperalgesia (60.5 %) and hyperpathia (56.3) achieved a much better level of pain control following surgery. Donoff and Colin reported similar finding where sensory function was improved in 77 % patients with anesthesia, whereas pain relief was established in only 42 % of patients that presented with pain [14]. The duration of the painful neuropathy may also be important. Short-term painful neuropathies (<6 months) may indicate early neuroma formation, while long-term painful neuropathies (>6 months) may result in central cortical changes that are not amenable to peripheral nerve repair. As a result, surgical management addressed at decreasing painful neuropathic pain may be more successful for early dysesthesia as opposed to late dysesthesia.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


