Armamentarium
|
History of the Procedure
The restoration of form and function to the maxillofacial complex has always been a challenge. This region is the most complex regarding function in the human body, with the airway and gastrointestinal system traversing its structures, and the skeleton providing the framework for the dentition and oral cavity; this area is critical for esthetics, speech, breathing, and nutritional intake. Failure to reconstruct maxillofacial defects after trauma or oncologic resection results in unopposed muscle action leading to collapse to the ipsilateral side, cosmetic asymmetry, loss of function, and decreased quality of life.
The need for immediate reconstruction of head and neck defects resulted in the development of the deltopectoral flap and pectoralis major flap, but immediate bony reconstruction for complex oncologic defects was not performed in the head and neck. The reconstruction of complex head and neck oncologic defects was revolutionized with microvascular free tissue transfer and the ability to immediately reconstruct mandibular defects.
In 1971, Strauch and coworkers were isolating a rib on a vascular pedicle and transposing this to the mandible in dogs as an island flap, and in 1974 Östrup and Fredrickson described microvascular rib transfer to the mandible in dogs. In 1973, Daniel and Taylor introduced the clinical reality of a free flap with an iliofemoral island flap transposed to the distal extremity. Taylor, in 1975, described the first free fibula flap transfer in humans, explaining the repair of traumatic injuries to the tibia in two patients. In 1977, the first free flap reconstruction of the mandible using a vascularized rib occurred, and Chen and Yan, in 1979, described the first osteocutaneous free fibula flap transferred to treat the traumatic loss of the radius. In the same year, Gilbert introduced the lateral approach to the fibula, which was more efficient compared to Taylor’s posterior approach, which required the patient to be in a prone position. Ten years would pass until Hidalgo, in 1989, introduced the osteocutaneous fibula free flap for use in mandibular reconstruction when he presented 12 cases of segmental mandibular defects averaging 13.5 cm.
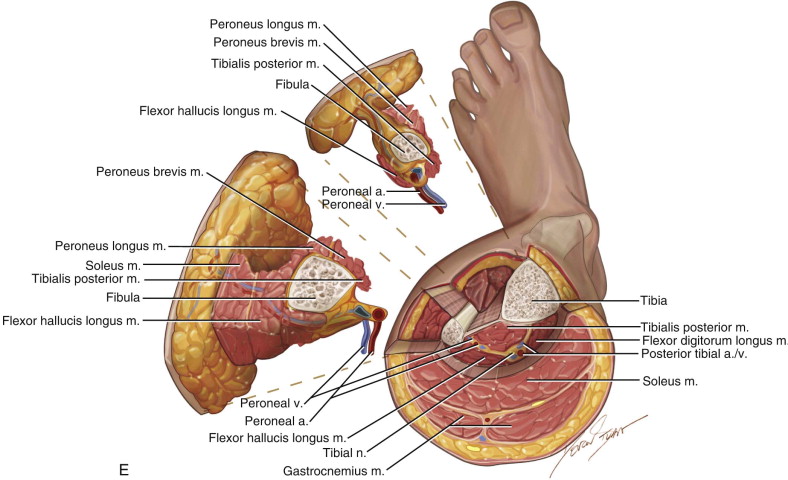
Since then, the fibula free flap has become the gold standard for reconstruction of a large variety of mandibular defects due to its consistency in size, vascular pedicle length, vessel diameter, as well as the ability to incorporate a reliable skin paddle with the bone flap. Wei and colleagues, in 1986, published an anatomic and clinical study showing the reliability of the osteocutaneous fibula free flap. The perforator anatomy has been revisited numerous times, confirming four to eight perforators along the fibula, most commonly located along the junction of the middle third and distal third of the fibula. These are more commonly septocutaneous, with the proximal perforators more likely to be musculocutaneous, traversing through the soleus or flexor hallucis longus muscle. The diameter of the peroneal artery ranges from 1 to 2.5 mm and the width of the venae comitantes ranges from 2 to 4 mm with a total pedicle length of up to 15 cm. The bone dimensions may range from a width of 1 to 3 cm, averaging 2 cm, with a length up to 26 cm. The skin paddle can be made up to a length of 32 cm and a width of 14 cm.
Many authors have described the reliability and viability of the fibula free flap with a success rate greater than 95%. Hidalgo showed the reliability more than a decade after surgery with 70% of patients tolerating a regular diet with maintenance of good esthetic outcomes.
Alternatives to the fibula free flap are also available, including nonvascularized corticocancellous bone grafts, vascularized scapula free flaps, osteocutaneous radial forearm free flaps, as well as the vascularized iliac crest free flap. Many contend that nonvascularized bone grafts offer better anatomic reconstructions with superior alveolar bone height, symmetric arch form, and graft width, whereas others state that the risk of infection, failure rates approaching 50%, and overall complication rates nearing 70% with nonvascularized corticocancellous bone grafts all make the approach too risky.
Frodel and coworkers and Moscoso and coworkers both compared the bone thickness and stock from four free flap donor sites—the iliac crest, scapula, fibula, and radius—and found that the iliac crest proved to be the most reliable implant donor site followed by the scapula, fibula, and radius; however, the difference between the iliac crest and scapula and between the scapula and fibula did not achieve statistical significance. The iliac crest has several disadvantages, such as having a bulky soft tissue cuff, difficult to inset, short vascular pedicle, the risk of postoperative hernia, and an inherent shape that sometimes makes contouring to the anterior mandible difficult. The disadvantages of the scapula include the lack of segmental blood supply, which does not tolerate osteotomies, the inability to use a two-team approach, and the risk of weakness and loss of range of motion associated with harvesting this flap. The radius provides a thin monocortical bone segment that does not tolerate osteotomies well, and the radius bone at the donor site is at risk for postoperative fractures.
Given its advantages, in many cases, the fibula free flap provides the best option for mandibular reconstruction. Its long bicortical bone, which is consistently uniform in terms of width and length, allowing reconstruction of extensive mandibular defects including the condyle, its consistent pedicle length and diameter with dual medullary and periosteal blood supply, the ability to incorporate a skin and muscular component, and the ability to use a two-team approach all make this the free flap of choice for most extensive mandibular reconstructions.
History of the Procedure
The restoration of form and function to the maxillofacial complex has always been a challenge. This region is the most complex regarding function in the human body, with the airway and gastrointestinal system traversing its structures, and the skeleton providing the framework for the dentition and oral cavity; this area is critical for esthetics, speech, breathing, and nutritional intake. Failure to reconstruct maxillofacial defects after trauma or oncologic resection results in unopposed muscle action leading to collapse to the ipsilateral side, cosmetic asymmetry, loss of function, and decreased quality of life.
The need for immediate reconstruction of head and neck defects resulted in the development of the deltopectoral flap and pectoralis major flap, but immediate bony reconstruction for complex oncologic defects was not performed in the head and neck. The reconstruction of complex head and neck oncologic defects was revolutionized with microvascular free tissue transfer and the ability to immediately reconstruct mandibular defects.
In 1971, Strauch and coworkers were isolating a rib on a vascular pedicle and transposing this to the mandible in dogs as an island flap, and in 1974 Östrup and Fredrickson described microvascular rib transfer to the mandible in dogs. In 1973, Daniel and Taylor introduced the clinical reality of a free flap with an iliofemoral island flap transposed to the distal extremity. Taylor, in 1975, described the first free fibula flap transfer in humans, explaining the repair of traumatic injuries to the tibia in two patients. In 1977, the first free flap reconstruction of the mandible using a vascularized rib occurred, and Chen and Yan, in 1979, described the first osteocutaneous free fibula flap transferred to treat the traumatic loss of the radius. In the same year, Gilbert introduced the lateral approach to the fibula, which was more efficient compared to Taylor’s posterior approach, which required the patient to be in a prone position. Ten years would pass until Hidalgo, in 1989, introduced the osteocutaneous fibula free flap for use in mandibular reconstruction when he presented 12 cases of segmental mandibular defects averaging 13.5 cm.
Since then, the fibula free flap has become the gold standard for reconstruction of a large variety of mandibular defects due to its consistency in size, vascular pedicle length, vessel diameter, as well as the ability to incorporate a reliable skin paddle with the bone flap. Wei and colleagues, in 1986, published an anatomic and clinical study showing the reliability of the osteocutaneous fibula free flap. The perforator anatomy has been revisited numerous times, confirming four to eight perforators along the fibula, most commonly located along the junction of the middle third and distal third of the fibula. These are more commonly septocutaneous, with the proximal perforators more likely to be musculocutaneous, traversing through the soleus or flexor hallucis longus muscle. The diameter of the peroneal artery ranges from 1 to 2.5 mm and the width of the venae comitantes ranges from 2 to 4 mm with a total pedicle length of up to 15 cm. The bone dimensions may range from a width of 1 to 3 cm, averaging 2 cm, with a length up to 26 cm. The skin paddle can be made up to a length of 32 cm and a width of 14 cm.
Many authors have described the reliability and viability of the fibula free flap with a success rate greater than 95%. Hidalgo showed the reliability more than a decade after surgery with 70% of patients tolerating a regular diet with maintenance of good esthetic outcomes.
Alternatives to the fibula free flap are also available, including nonvascularized corticocancellous bone grafts, vascularized scapula free flaps, osteocutaneous radial forearm free flaps, as well as the vascularized iliac crest free flap. Many contend that nonvascularized bone grafts offer better anatomic reconstructions with superior alveolar bone height, symmetric arch form, and graft width, whereas others state that the risk of infection, failure rates approaching 50%, and overall complication rates nearing 70% with nonvascularized corticocancellous bone grafts all make the approach too risky.
Frodel and coworkers and Moscoso and coworkers both compared the bone thickness and stock from four free flap donor sites—the iliac crest, scapula, fibula, and radius—and found that the iliac crest proved to be the most reliable implant donor site followed by the scapula, fibula, and radius; however, the difference between the iliac crest and scapula and between the scapula and fibula did not achieve statistical significance. The iliac crest has several disadvantages, such as having a bulky soft tissue cuff, difficult to inset, short vascular pedicle, the risk of postoperative hernia, and an inherent shape that sometimes makes contouring to the anterior mandible difficult. The disadvantages of the scapula include the lack of segmental blood supply, which does not tolerate osteotomies, the inability to use a two-team approach, and the risk of weakness and loss of range of motion associated with harvesting this flap. The radius provides a thin monocortical bone segment that does not tolerate osteotomies well, and the radius bone at the donor site is at risk for postoperative fractures.
Given its advantages, in many cases, the fibula free flap provides the best option for mandibular reconstruction. Its long bicortical bone, which is consistently uniform in terms of width and length, allowing reconstruction of extensive mandibular defects including the condyle, its consistent pedicle length and diameter with dual medullary and periosteal blood supply, the ability to incorporate a skin and muscular component, and the ability to use a two-team approach all make this the free flap of choice for most extensive mandibular reconstructions.
Indications for the Use of the Procedure
The goal of maxillofacial reconstruction using the fibula free flap is to not only restore continuity to the mandible or maxilla but also to restore form and function, allowing a return to normal speech, chewing, and swallowing. There are several indications for fibula reconstruction, such as oncologic surgery, allowing for aggressive resection and reliable reconstruction of previously unreconstructable defects. In these cases the fibula or other free tissue transfer has the significant advantage of withstanding postsurgical radiotherapy. Other indications include posttraumatic defects, such as the definitive management of gunshot wounds, osteoradionecrosis, osteomyelitis, and congenital deformities. Mandibular defects larger than 4 to 6 cm are typically used for a fibula flap, and this osseous flap surpasses all others when the anterior mandibular segment is involved, or for those requiring condylar reconstruction.
Preoperative Assessment
Preoperative assessment prior to harvesting a fibula free flap begins with the attainment of a thorough medical history and physical examination that may reveal poor reliability of the peroneal artery and venae comitantes. This includes prior trauma, deep vein thrombosis, or peripheral vascular disease as well as diabetes and hypertension. Signs of previous trauma or surgery or atypical skin temperature, skin lesions, and hair growth should be examined for evidence of peripheral vascular disease.
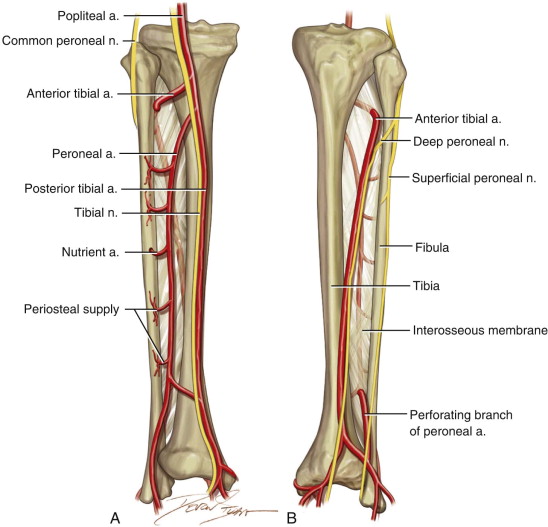
Vascular studies have become extremely useful in preoperative preparation for free fibula harvest. Some groups have advocated that thorough physical examination and noninvasive Doppler examination are sufficient, whereas others have concluded that preoperative angiography with its high positive predictive value and sensitivity can improve the chances of a successful outcome. Kim and colleagues reviewed the variations in 1000 femoral arteriograms, and normal branching of the popliteal artery was present in 92.2%. Of the 7.8% variations, most had a high origin of the anterior tibial artery or a trifurcation pattern. Of the variant patterns to the foot, 5.6%, the most common, reflected the supply to the distal posterior tibial artery from the peroneal artery. In 0.2% to 8% of the population, the peroneal artery is the dominant blood supplier to the foot, also known as peroneal artery magna, secondary to the absence or hypoplasia of the anterior and posterior tibial arteries.
Conventional angiography evaluation of the lower extremity for the fibula free flap has been associated with risks of hemorrhage and thrombosis from the arterial catheterization, and has now evolved into less invasive studies such as computed tomography angiography (CTA), magnetic resonance angiography (MRA), and color Doppler flow examinations. CTA and MRA are comparable except that a CTA is faster than an MRA and exposes the patient to radiation. Both are able to image the trifurcation of vessels and are able to image perforators. On the other hand, Doppler ultrasound is operator dependent and unable to image the trifurcation of vessels. MRA also cannot be used for patient-specific modeling during virtual surgical planning.
Technique: Osteocutaneous Fibula Harvest
Step 1:
Patient Positioning and Preparation
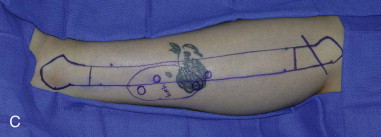
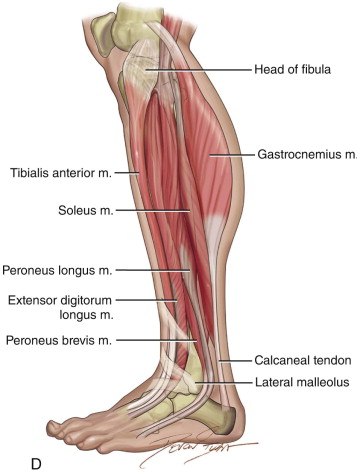
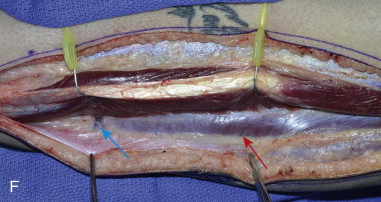
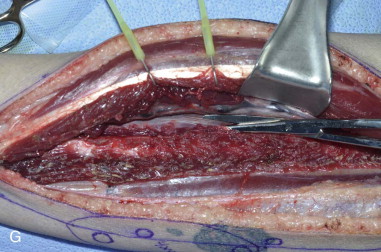
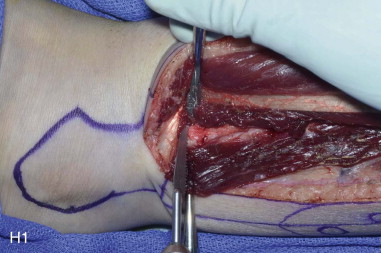
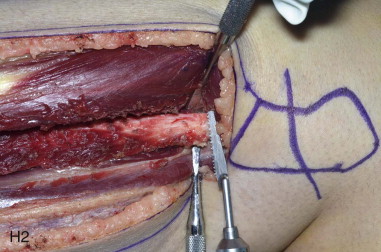
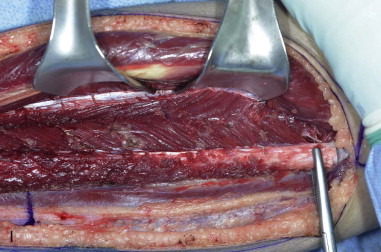
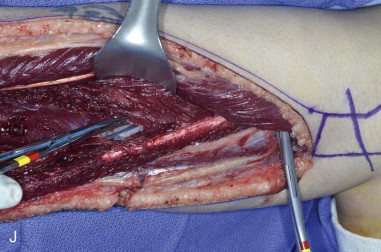
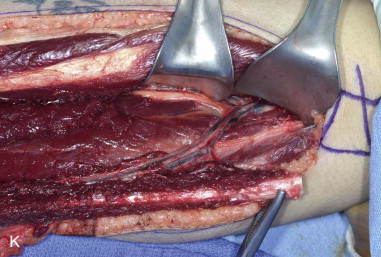
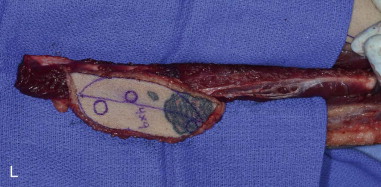
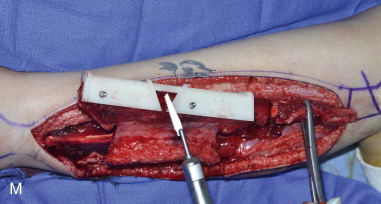
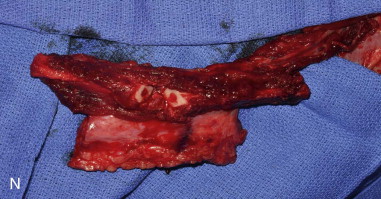
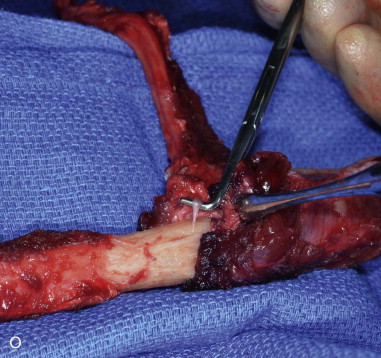
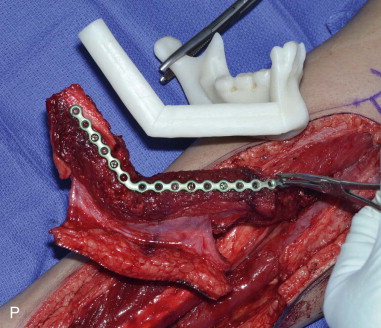
The patient’s leg is positioned so that it is supported by a hip bump internally rotating the leg and pelvis with knee and foot support, which positions the leg with a 90-degree bend at the knee. The toes may be prepped or covered, but exposure of the foot for Doppler assessment of the posterior tibial artery and dorsalis pedis pulses allows for confirmation of vascular flow to the foot throughout the procedure. The topographic anatomy is marked with a line from the fibular head superiorly and the lateral epicondyle of the ankle inferiorly. This line approximates the intermuscular septum. Two lines at 90 degrees are marked 6 to 8 cm above the inferior and below the ends of prior marking, which denotes bone that will be spared for ankle and knee joint stability. The superior line also protects the peroneal nerve, which passes 1 to 2 cm below the fibular head. A Doppler may be utilized to mark potential perforators for inclusion in the skin paddle. It should be noted that harvest of the fibula and skin more distally generates a longer vascular pedicle, which may be of importance particularly when the contralateral neck vessels are to be utilized for anastomosis. Generally, the dominant septocutaneous perforators are located more distally, allowing the centering of the flap in the region of the distal and middle thirds of the leg ( Figure 115-1, A-C ).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


