In 2000, the US Food and Drug Administration (FDA) approved the use of 4% articaine with epinephrine 1:100,000, and with epinephrine 1:200,000 in 2006. Articaine has been commonly compared with its predecessor, lidocaine hydrochloride. Since its introduction in 1948, lidocaine has maintained a status as the most widely used local dental anesthetic in most countries. Proven efficacy with low allergenicity and toxicity over long-term clinical use and research have confirmed the value and safety of this drug. Thus, it became the gold standard to which all new local anesthetics are compared. Despite the gold standard status of lidocaine, numerous reports and editorials have supported and recognized the use of articaine.
In 1969, carticaine hydrochloride, with a chemical code name of Hoe 40 045, was synthesized as the first amide-type drug with a lipophilic thiophene ring and an additional ester side chain ( Fig. 1 ). Carticaine became available for clinical use in Germany in 1976, and in 1984 was renamed articaine . In 2000, the US Food and Drug Administration (FDA) approved the use of 4% articaine with epinephrine 1:100,000, and with epinephrine 1:200,000 in 2006.
Articaine has been commonly compared with its predecessor, lidocaine hydrochloride ( Fig. 2 ). Since its introduction in 1948, lidocaine has maintained a status as the most widely used local dental anesthetic in most countries. Proven efficacy with low allergenicity and toxicity over long-term clinical use and research have confirmed the value and safety of this drug. Thus, it became the gold standard to which all new local anesthetics are compared. Several injectable lidocaine formulations have been approved by the FDA for dental applications: 2% without vasoconstrictor, 2% with epinephrine 1:50,000, and 2% with epinephrine 1:100,000. The 2% lidocaine with 1:100,000 epinephrine formulation continues to be the most commonly used local anesthetic agent for routine dental procedures in the United States.
Despite the gold standard status of lidocaine, numerous reports and editorials have supported and recognized the use of articaine. An editorial in the Journal of the American Dental Association acknowledged that articaine “has garnered a majority of the dental market in many of the countries in which it is available.” Another editorial quoted a drug company’s marketing approach, “Articaine has become the most popular local anesthetic for dentists wherever it has been introduced.”
In a 1993 survey based in Ontario, Canada, dentists reported articaine with epinephrine 1:200,000 was used 19.9% of the time, articaine with epinephrine 1:100,000 was used 17.9% of the time, and lidocaine with epinephrine 1:100,000 was used 23.4% of the time. In a 2007 follow-up survey based in Ontario, Canada, dentists reported articaine with epinephrine 1:200,000 was used 27.04% of the time, articaine with epinephrine 1:100,000 was used 17.16% of the time, and lidocaine with epinephrine 1:100,000 was used 37.31% of the time. In a survey from Germany, 911 dentists reported the use of articaine 90% of the time and lidocaine 2% of the time. Another survey representing 541 dentists in Germany indicated routine administration of articaine 72% of the time and lidocaine 13% of the time.
Articaine’s superior reputation has been primarily based on clinicians’ opinion that it may possess enhanced diffusion properties and better anesthetic efficacy. In a standard textbook of local anesthesia, articaine was described as having potency 1.5 times that of lidocaine, faster onset, and increased success rate, with dentists reporting that they “don’t miss as often.” A review article on articaine clinical pharmacology reported, “In dentistry, articaine is the drug of choice in the vast majority of literature.” Isen claimed “enhanced action of articaine over other local anesthetics” based on the molecule having more lipid-soluble abilities across the nerve membrane. Many of these claims may have been based on speculation because most of the literature has failed to support these claims.
Reports of articaine’s superiority were mainly founded on the notion that its thiophene ring bestows enhanced performance. This feature has been credited with providing increased lipid solubility and protein binding, two properties theoretically related to increased anesthetic efficacy. Lipid solubility is an intrinsic quality of local anesthetic potency. This quality is essential for penetration of the anesthetic through the lipid nerve membrane and subsequent diffusion into surrounding tissues. In a clinical study evaluating this concept, alveolar blood levels were measured post-extraction for anesthetic concentrations (and their byproducts). After the injection of 2 ml of either 4% articaine or 2% lidocaine, both with epinephrine 1:100,000, a significant two-fold higher mean of articaine was observed in alveolar blood. The rationale for this “better diffusion” after injection was based on the higher descent of concentration derived from articaine. Therefore, superior anesthetic diffusion properties of articaine were not confirmed in the research.
The degree of anesthetic molecules binding to the nerve membrane was suggested to dictate the duration of the anesthetic effect. The more secure a bond is, the slower the anesthetic is released from the receptor sites in the sodium channels and the greater the duration of the anesthetic effect. As determined by Courtney and colleagues, mere lipid solubility of a local anesthetic did not determine the action on the ionic channels. Instead, Uihlein determined that binding properties of the local anesthetic agent to plasma proteins have a greater correlation to action on ionic channels than does lipid solubility.
The first clinical trial in dentistry testing the efficacy of articaine was conducted in Denmark in 1972 by Winther and Nathalang. Comparisons were made of 2% articaine with and without epinephrine 1:200,000, 2% lidocaine with and without epinephrine 1:200,000, and other anesthetic compounds. The results showed that articaine with epinephrine was significantly superior to lidocaine with epinephrine (and to the other anesthetic compounds) with respect to “frequency, extent and duration of analgesia.”
Muschaweck and Rippel conducted an early investigation of the pharmacology and toxicology of articaine (0.05%–0.5% solutions) in animal experiments, with lidocaine (0.05%–0.5% solutions) as a comparison. This investigation found that when compared with lidocaine, articaine had 1.5 times higher anesthetic activity in conduction anesthesia infiltration, “markedly superior” efficacy in infiltration anesthesia, equivalent efficacy in topical anesthesia, and similar low toxicity to local tissues.
In a multicenter trial conducted on 1325 subjects (aged 4–80 years) comparing the safety and efficacy of 4% articaine and 2% lidocaine, both with epinephrine 1:100,000, both agents demonstrated clinically effective and appropriate local anesthesia during general dental procedures.
A literature review of articaine and lidocaine
To date, 27 publications have reported on clinical trials comparing the anesthetic efficacy of 4% articaine with 2% lidocaine, both with epinephrine 1:100,000 in dental applications. Although differences between these formulations were not consistently reported, a common trend is seen for articaine to outperform the gold standard lidocaine in dental applications. However, statistically speaking, results were divided regarding the anesthetic efficacy of the two anesthetic agents. Overall, 12 of these publications reported a statistically significant difference between the anesthetic agents, whereas 12 other studies found no statistical significant difference. Two studies reported a contrast in statistical significance between these anesthetic agents, depending on the tooth type analyzed. One study did not statistically analyze the results; however, the outcome data followed the trend for articaine to be somewhat better than lidocaine. In more recent publications, articaine efficacy has been tested when used as a supplemental anesthetic or in supplemental injection techniques.
A quantitative meta-analysis comparing articaine and lidocaine
Because of the disparity in the results of published clinical trials, and because many studies lacked sufficient subject numbers to independently support a difference of statistical significance, the anesthetic efficacy of articaine is unclear. Using meta-analysis to quantitatively synthesize results from multiple clinical studies comparing 4% articaine with 2% lidocaine, both with epinephrine 1:100,000, a statistical comparison was performed.
The details of this quantitative review were published online in 2008 as one of the author’s master’s thesis. The methods and results are summarized later. These specific formulations (anesthetic and epinephrine) were selected based on their frequent dental use in both clinical and research arenas. The primary outcome of interest was a comparison of anesthetic success achieved from the anesthetic agents as defined independently in each study.
A literature search to locate all published research was initially performed. The MeSH database, the National Library of Medicine’s controlled vocabulary indexing system, was used to search the following terms closely related to this study: carticaine , articaine , lidocaine , lignocaine , local anesthetics , and dental anesthesia . It was concluded that the most appropriate and exhaustive Medical Subject Heading terms for the purpose of searching PubMed were carticaine and lidocaine . Searching PubMed, the National Library of Medicine’s text-based search and retrieval system for biomedical literature, through January 2008 using the combination of MeSH terms carticaine and lidocaine produced 76 publications. Through limiting the literature to human studies, the 76 articles were reduced to 64. Abstracts or manuscripts were obtained and analyzed for these 64 studies.
Starting with these 64 identified articles, the first group of exclusions included 19 studies based on nondental topics. Of the remaining 45 dental-related articles, 10 were either review or editorial publications and therefore excluded. Furthermore, 14 articles could be eliminated because they reported on local anesthesia topics other than efficacy, such as allergic reactions, tissue reactions, anxiety, injection pain, systemic factors, complications, or nerve injury. One study only evaluated the efficacy of articaine and did not include lidocaine. Four studies evaluated both anesthetic agents, but the specified concentrations of one or both agents were not tested.
At this point, the remaining 16 articles were all clinical trials comparing the specific anesthetic agents of interest in dental applications. Supplemental anesthesia, with only lidocaine given initially, was reported in one publication. Because no comparison of initial anesthetic administration was made, this study was excluded. Further exclusions included two studies comparing the anesthetic agents of interest; however, randomization was not reported in one, and the methodology and results were unclear in another. Another publication was excluded because it reported data included in a previously included publication. After this level of exclusion, 12 original articles remained of human randomized clinical studies evaluating the dental anesthetic efficacy of both 4% articaine and 2% lidocaine, both with epinephrine 1:100,000, as initial local anesthetics. To exhaust the search for publications fulfilling the criteria stated earlier, cross-citations of these 12 studies were explored.
The final step in determining inclusion was the availability of sufficient information for analytic evaluation. For four of the 12 studies, it was necessary to seek supplemental data to include these studies in the proposed meta-analytic comparisons. The shortcomings of these four studies pertained to the lack of information relating to absolute determination of anesthetic success and failure in subjects. In particular, the information necessary for this meta-analysis included the number of experimental applications with absolute anesthetic success and absolute anesthetic failure with percentage calculations and P values. The results of three of the studies reported timeline comparisons of anesthetic onset and duration only. No outcome data were available to calculate a percentage of anesthetic success and failure. Another study only reported the mean, median, and range of visual analog scale scores for each anesthetic agent given. Thus, the results failed to define or report anesthetic success in terms of subjects who were pain-free.
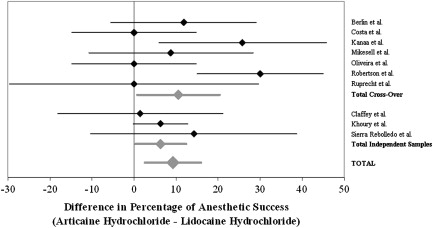
Although not reported, the supplemental data necessary for this meta-analysis would most likely have been recorded during the course of each study. Reasonable effort was made to contact the authors of these four studies to obtain the necessary supplemental information. Adequate unpublished data were successfully obtained from Costa and colleagues and Oliveira and colleagues. Therefore, 10 studies could be included in this meta-analysis ( Table 1 ). Inclusion criteria specified randomized clinical trials with clear determination of anesthetic efficacy with respect to initial administration of these two anesthetic agents in dental applications, resulting in total 1146 subjects and 1395 anesthetic administrations.
| Study | Study Type | n a | Difference in Percentages | 95% CI (Lower %, Upper %) |
|---|---|---|---|---|
| Berlin et al | CO | 102 | 11.77% | (−5.59%, 29.12%) |
| Costa et al | CO | 40 | 0% | (−14.80%, 14.80%) |
| Kanaa et al | CO | 62 | 25.81% | (5.93%, 45.68%) |
| Mikesell et al | CO | 114 | 8.77% | (−10.70%, 28.25%) |
| Oliveira et al | CO | 40 | 0% | (−14.80%, 14.80%) |
| Robertson et al | CO | 120 | 30.0% | (15.02%, 44.98%) |
| Ruprecht and Knoll-Kohler | CO | 20 | 0% | (−29.60%, 29.60%) |
| Claffey et al | IS | 72 | 14.7% | (−18.15%, 21.08%) |
| Khoury et al | IS | 771 | 6.37% | (−0.11%, 12.85%) |
| Sierra Rebolledo et al | IS | 54 | 14.17% | (−10.41%, 38.75%) |
As indicated in Table 1 , two of these studies reported significantly superior performance of articaine compared with lidocaine based on achievement of pulpal anesthetic success; a third study reported a suggestive result ( P = .057). In four additional studies, the observed rate of anesthetic success was greater for articaine, although not significantly so. The performance of the two anesthetic agents was identical in the remaining three studies.
As shown in Fig. 3 , the combined estimate of the difference obtained using a random effects meta-analytic model was associated with an estimated 9.21% greater proportion of anesthetic success with articaine than with lidocaine (95% CI, 2.56%–15.58%), providing evidence of articaine superiority.
A quantitative meta-analysis comparing articaine and lidocaine
Because of the disparity in the results of published clinical trials, and because many studies lacked sufficient subject numbers to independently support a difference of statistical significance, the anesthetic efficacy of articaine is unclear. Using meta-analysis to quantitatively synthesize results from multiple clinical studies comparing 4% articaine with 2% lidocaine, both with epinephrine 1:100,000, a statistical comparison was performed.
The details of this quantitative review were published online in 2008 as one of the author’s master’s thesis. The methods and results are summarized later. These specific formulations (anesthetic and epinephrine) were selected based on their frequent dental use in both clinical and research arenas. The primary outcome of interest was a comparison of anesthetic success achieved from the anesthetic agents as defined independently in each study.
A literature search to locate all published research was initially performed. The MeSH database, the National Library of Medicine’s controlled vocabulary indexing system, was used to search the following terms closely related to this study: carticaine , articaine , lidocaine , lignocaine , local anesthetics , and dental anesthesia . It was concluded that the most appropriate and exhaustive Medical Subject Heading terms for the purpose of searching PubMed were carticaine and lidocaine . Searching PubMed, the National Library of Medicine’s text-based search and retrieval system for biomedical literature, through January 2008 using the combination of MeSH terms carticaine and lidocaine produced 76 publications. Through limiting the literature to human studies, the 76 articles were reduced to 64. Abstracts or manuscripts were obtained and analyzed for these 64 studies.
Starting with these 64 identified articles, the first group of exclusions included 19 studies based on nondental topics. Of the remaining 45 dental-related articles, 10 were either review or editorial publications and therefore excluded. Furthermore, 14 articles could be eliminated because they reported on local anesthesia topics other than efficacy, such as allergic reactions, tissue reactions, anxiety, injection pain, systemic factors, complications, or nerve injury. One study only evaluated the efficacy of articaine and did not include lidocaine. Four studies evaluated both anesthetic agents, but the specified concentrations of one or both agents were not tested.
At this point, the remaining 16 articles were all clinical trials comparing the specific anesthetic agents of interest in dental applications. Supplemental anesthesia, with only lidocaine given initially, was reported in one publication. Because no comparison of initial anesthetic administration was made, this study was excluded. Further exclusions included two studies comparing the anesthetic agents of interest; however, randomization was not reported in one, and the methodology and results were unclear in another. Another publication was excluded because it reported data included in a previously included publication. After this level of exclusion, 12 original articles remained of human randomized clinical studies evaluating the dental anesthetic efficacy of both 4% articaine and 2% lidocaine, both with epinephrine 1:100,000, as initial local anesthetics. To exhaust the search for publications fulfilling the criteria stated earlier, cross-citations of these 12 studies were explored.
The final step in determining inclusion was the availability of sufficient information for analytic evaluation. For four of the 12 studies, it was necessary to seek supplemental data to include these studies in the proposed meta-analytic comparisons. The shortcomings of these four studies pertained to the lack of information relating to absolute determination of anesthetic success and failure in subjects. In particular, the information necessary for this meta-analysis included the number of experimental applications with absolute anesthetic success and absolute anesthetic failure with percentage calculations and P values. The results of three of the studies reported timeline comparisons of anesthetic onset and duration only. No outcome data were available to calculate a percentage of anesthetic success and failure. Another study only reported the mean, median, and range of visual analog scale scores for each anesthetic agent given. Thus, the results failed to define or report anesthetic success in terms of subjects who were pain-free.
Although not reported, the supplemental data necessary for this meta-analysis would most likely have been recorded during the course of each study. Reasonable effort was made to contact the authors of these four studies to obtain the necessary supplemental information. Adequate unpublished data were successfully obtained from Costa and colleagues and Oliveira and colleagues. Therefore, 10 studies could be included in this meta-analysis ( Table 1 ). Inclusion criteria specified randomized clinical trials with clear determination of anesthetic efficacy with respect to initial administration of these two anesthetic agents in dental applications, resulting in total 1146 subjects and 1395 anesthetic administrations.



