Introduction
The purposes of this research were to investigate the long-term responses of mandibular condylar cartilage to experimentally induced disordered occlusion and to evaluate changes in the expression of the SDF-1/CXCR4 axis.
Methods
Experimentally induced disordered occlusions were created in 8-week-old female Sprague-Dawley rats by orthodontic methods. After 24 weeks, remodeling of the mandibular condylar cartilage was assessed by hematoxylin and eosin staining. Protein and mRNA expression of SDF-1, CXCR4, MMP9, IL6, OPG, and RANKL were investigated by means of immunohistochemical staining and real-time polymerase chain reaction.
Results
Obvious cartilage degenerative remodeling responses were observed; they appeared as uneven distributions of cellular disposition, loss of cartilage surface integrity, and cell-free areas. Regenerative responses presenting as thickening of the whole and the calcified cartilage layers in the experimental group were also observed. Compared with the age-matched controls, the protein and mRNA levels of SDF-1, CXCR4, MMP9, IL6, and OPG, but not RANKL, were increased in the experimental group (all, P <0.05). In addition, the mRNA level of RANKL/OPG showed a decreasing trend in the experimental group compared with the age-matched controls ( P = 0.052).
Conclusions
This study demonstrated that long-term experimentally induced disordered occlusion leads to a combined response in degeneration and regeneration of mandibular cartilage, accompanied by active interaction of the SDF-1/CXCR4 axis and local upregulation of MMP9, IL6, and OPG.
The temporomandibular joint (TMJ) maintains the ability of active remodeling throughout life, and the mandibular condyle cartilage has highly sensitive and reconstructive potentials to repeated mechanical loading from dental occlusion. Thus, changes in occlusion, such as disordered occlusion, unilateral bite raise, loss of posterior teeth, incisor disocclusion, and continuous forward movement of the jaw, which cause changes in the biomechanical environment of the TMJ, lead to pathologic or physiologic remodeling of the condyle. Under normal circumstances, condylar chondrocytes maintain a balance between anabolic and catabolic activities for preservation of the structural and functional integrity of the condylar cartilage. Chondrocytes express various cytokines and chemokines, which are involved in the remodeling of the TMJ in response to functional demands. When functional demands exceed the adaptive capacity of the TMJ, production of matrix metalloproteinases (MMPs) and degenerative factors such as proinflammatory molecules by chondrocytes increase considerably, resulting in aberrant cartilage destruction.
There are many studies on the effects of multiple catabolic factors on the development of osteoarthritis, which is a problem of homeostasis imbalance. Active remodeling activities maintain the balance between degeneration and regeneration. However, the complex cytokine network involved in that process remains unclear and deserves further investigation. Chemokines are soluble peptides that regulate the movement, morphology, proliferation, and differentiation of cells. Stromal cell-derived factor-1 (SDF-1) is an 8-kDa peptide originally isolated from a bone marrow stromal cell line; it belongs to the CXC subfamily of chemokines and acts as a key chemokine for stem cells. Unlike other chemokines, SDF-1 achieves regulation by binding solely to its receptor, CXCR4, which belongs to a family of 7 transmembrane G-protein coupled receptors. Recent studies have reported that SDF-1 secreted by bone marrow cells adjacent to the cartilage elicits functional effects through its specific receptor CXCR4 on the surface of chondrocytes. The interaction of SDF-1 and CXCR4 not only elicits catabolic processes in osteoarthritis cartilage by inducing the release of interleukin-6 (IL6) and MMPs, but also plays an important role in regulating the proliferative activity of osteoarthritis chondrocytes. In addition, receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG) are produced by articular chondrocytes in the superficial zone of normal cartilage, whereas their expression was found to extend to the middle zone in osteoarthritis. Increased RANKL/OPG ratio but decreased OPG-positive cells were detected in the osteoarthritic knee and TMJ ; these might influence cartilage turnover and cause subchondral bone changes. Evidence showed that OPG plays a crucial role in regulating cartilage metabolism because OPG-deficient mice demonstrate severe destruction of growth plate cartilage and markedly thinned cartilage layers and invasion of the vasculature into the calcified layer. Local intra-articular administration of human OPG to articular chondrocytes and adeno-associated virus-mediated human OPG to synovial tissues protects against the degeneration of the cartilage and bone of a murine knee joint.
In a series of previous studies, we have reported a rat model with a specially designed occlusal abnormality that highly resembled clinical occlusal problems. Typical TMJ osteoarthritis-like changes were induced at 8 and 12 weeks; this is a sign of enhanced remodeling of the TMJ upon imbalance between anabolism and catabolism in the osteochondral junction. The coincident occurrence of cartilage destruction and chondrocyte proliferation in this TMJ osteoarthritis model is attractive. To better understand the features of long-term condylar adaptation to a disordered occlusion, we investigated the cartilage response in this rat model after 24 weeks. We investigated the histologic changes, as well as changes of the distribution pattern of SDF-1 and its receptor CXCR4, markers of cartilage destruction (IL6 and MMP9), and markers of endochondral ossification (OPG and RANKL) in the mandibular condyle.
Material and methods
Twelve 8-week-old female Sprague–Dawley rats (weight, 180-190 g) were provided by the animal center of the Fourth Military Medical University in Xi’an, China. All procedures and the care for the rats were approved by the university’s ethics committee (no. 2009-158) and performed according to institutional guidelines. The rats were allotted randomly into experimental groups or control groups ( n = 6). Disordered occlusions were created as previously described. Briefly, an orthodontic elastic rubber band (1/8#; 3M Unitek, Monrovia, Calif) was inserted between the maxillary left first and second molars and between the mandibular right first and second molars so that the elastic force gradually moved the first molars medially. Seven days later, a gap about 0.8 mm wide was created. Then the rubber bands were replaced by self-curing resin (Zhangjiang Biomaterial, Shanghai, China) to maintain this gap until the end of the experiment. Four weeks later, the same method was used to distally move the maxillary left and mandibular right third molars. In this way, 2 first and 2 third molars were moved away from their original positions and no longer intercuspated with their opposite molars ( Fig 1 ). The control rats received the same procedure but without disturbing the occlusion. The rats were killed at the end of the 24th week after the beginning of the experiment. No rats showed any sign of disability, and all received the same standardized diet during the entire procedure.
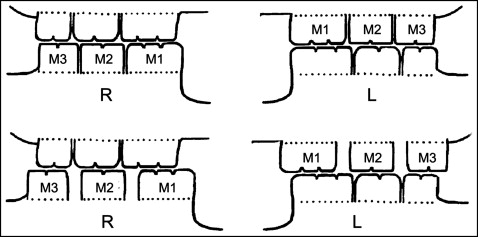
The right TMJ tissue blocks in each group were dissected out. After decalcification, the tissues were embedded in paraffin wax for histologic and immunohistochemical staining. Serial sections 5 μm thick were cut though the TMJ in the sagittal plane by using a rotary microtome (RM2135; Leica Microsystems, Nussloch, Germany). To ensure a reliable comparison between the specimens from different subgroups, the central sagittal sections of each joint were selected for this study. The condylar heads of the left joints were carefully isolated, and RNA extraction was performed for further real-time polymerase chain reaction (PCR) analysis.
Hematoxylin and eosin staining was used for the histologic assessments. As previously reported, the surface of the condylar cartilage, defined as the area between the anterior and posterior attachment points of the joint’s disc to the condyle, was equally divided into anterior, center, and posterior thirds. The thicknesses of the cartilage layer and the calcified cartilage layer were measured at the quartering points of the center and posterior thirds with a computer-assisted image analyzing system (Qwin Plus; Leica Microsystem Imaging Solutions, Cambridge, United Kingdom), and the average of the 6 values was taken as the thickness of the sample.
To detect the distribution of SDF-1, CXCR4, IL6, RANKL, MMP9, and OPG in the mandibular condyle, immunolocalization was used with 6 primary antibodies: anti-SDF-1 (rabbit polyclonal, sc-28876, 1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, Calif), anti-CXCR4 (rabbit polyclonal, Ab-2074, 1:50 dilution; Abcam Biochemicals, Cambridge, Mass), anti-MMP9 (goat polyclonal, sc-6840, 1:50 dilution; Santa Cruz Biotechnology), anti-IL6 (rabbit polyclonal, sc-1265, 1:50 dilution; Santa Cruz Biotechnology), anti-OPG (rabbit polyclonal, sc-8468, 1:50 dilution; Santa Cruz Biotechnology), and anti-RANKL (rabbit polyclonal, sc-7628, 1:50 dilution; Santa Cruz Biotechnology), respectively. Immunostaining procedures followed a standard 3-step avidin-biotin complex method as described previously. The percentages of immunopositive cell areas were measured as described previously. In brief, image acquisition was performed by a Leica system (DFC490; Leica, Wetzlar, Germany). Six squares (200 × 200 μm), covering the proliferative layer, the hypertrophic layer, the calcified cartilage layer, and part of the subchondral bone, were located at the quarter points of the center and posterior thirds of the condylar cartilage. The percentages of the areas of positive cells were determined in the sampling regions by using a computer-assisted image analyzing system (Qwin Plus) at the same threshold of staining. For the reading of each antibody staining, a uniform setting for all slides was applied. Negative controls were stained with nonimmune serum instead of primary antibody. Cartilage thickness and immunopositive cell numbers were measured on 3 central sagittal sections of each condyle, and the mean values were obtained.
Cartilage was carefully dissected from the mandibular condylar under a dissecting microscope (SZX9; Olympus, Tokyo, Japan) and used for RNA extraction, as we reported previously. Every 2 of 6 condylar cartilages in each subgroup were put together for homogenization in liquid nitrogen ( n = 3). Total RNA was extracted with Trizol Reagent (Invitrogen, Carlsbad, Calif), following the manufacturer’s instructions. GAPDH was used as the endogenous control, and the target gene primers are listed in the Table .
| Gene | Sequence | Size | Gene bank number |
|---|---|---|---|
| SDF-1 | F:5′ >GTGCCCTTCAGATTGTTGCAAGGC<3′ R:5′>CCTCAGGCGTCTGACTCACACC<3′ |
119bp | NM_001033882.1 |
| CXCR4 | F:5′ >CCATGGAAATATACACTTCGGA<3′ R:5′ >AATAGATGGTGGGCAGGAAG<3′ |
129bp | NM_022205.3 |
| MMP9 | F:5′>CGCTGGGCTTAGATCATTCT<3′ R:5′ >GAGCCACGACCATACAGATG<3′ |
124bp | NM_031055.1 |
| IL6 | F:5′ >CCACTTCACAAgTCggAggCTTA<3′ R:5′ >gTgCATCATCgCTgTTCATACAATC<3′ |
108bp | NM_012589.1 |
| RANKL | F:5′ >TCGGGTTCCCATAAAGTCAG<3′ R:5′ >CTTGGGATTTTGATGCTGGT<3′ |
204bp | XM_003503083.1 |
| OPG | F:5′ >TGGGAATGAAGATCCTCCAG<3′ R:5′ >GAGGAAGGAAAGGGCCTATG<3′ |
149bp | NM_012870.2 |
| GAPDH | F:5′ >GGCACAGTCAAGGCTGAGAATG<3′ R:5′ >ATGGTGGTGAAGACGCCAGTA<3′ |
142bp | NG_027842.1 |
Real-time PCR was performed in a reaction volume of 20 μL by using SYBR Premix Ex Taq II (Takara, Dalian, China). Assays were performed in triplicate on the PCR machine (7500 Real Time; Applied Biosystems, Foster City, Calif). The expression levels of target genes, relative to GAPDH, were calculated by the formula 2 −ΔΔCt . The results were calculated as the relative quantification of the target gene compared with the control group.
Statistical analysis
The measurement procedures were performed twice in a blinded fashion over an interval of 1 week by 2 independent observers (B.K. and Q.-Y.W.). There was a high level of agreement ( r >0.9) between the observers. The mean of the 2 measurements was used for further statistical analysis. The data in histology and immunohistochemistry analysis and the quantitative PCR analysis were presented as means ± standard errors for each group and analyzed by using SPSS software (version 12.0; SPSS, Chicago, Ill). All data acquisition and analyses were performed in a blinded fashion. The gene levels of RANKL/OPG rationed among the 12-week and 24-week control and experimental groups were compared by 1-way analysis of variance and the Student-Newman-Keuls post-test, and the other quantitative data for the control and experimental groups were compared with the Student t test. P values less than 0.05 were considered statistically significant.
Results
The experimentally created gaps were maintained until the end of the experiment, and thus the disordered molar occlusion relationships causing an unmatched convexto-concave occlusal relationship were present throughout the experimental period.
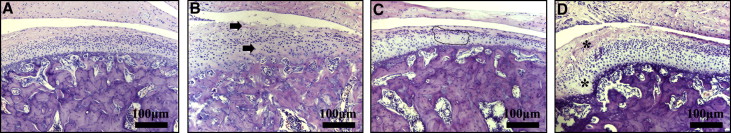
In the control group, the condylar cartilage appeared normal, and the fibrous, proliferative, hypertrophic chondrocyte and endochondral ossification layers could be clearly distinguished ( Fig 2 , A ). In contrast, the experimentally induced disordered occlusions (EIDO) group showed local pathologic changes in the middle and posterior thirds of the condylar cartilage, as previously described. The degenerative changes took the form of uneven distribution of cellular disposition, cell-free areas, and loss of cartilage surface integrity ( Fig 2 , B and C ). Additional proliferative changes were also observed, characterized as local thickening of the fibrous layer and conjunctive invaginations penetrating into the subchondral bone ( Fig 2 , D ). Moreover, the thicknesses of the whole cartilage layer and the calcified cartilage layer in the experimental group increased significantly compared with those in the control group ( P <0.05).

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


