Introduction
Self-ligating orthodontic brackets rely on clips, rather than ligatures, to hold the archwire in place. It is unknown whether replacing ligatures with clips affects the adherence of Streptococcus mutans . The aim of this research was to evaluate whether self-ligating brackets have an advantage over conventional brackets as determined by the adherence of S mutans .
Methods
The sample consisted of 50 esthetic brackets, divided into 3 experimental groups and 2 control groups of 10 brackets each. Two experimental groups were active self-ligating brackets (QuicKlear; Forestadent, Pforzheim, Germany; and In-Ovation C; Dentsply GAC, Bohemia, NY); the other was a passive self-ligating bracket (Damon 3; Ormco, Glendora, Calif). The 2 control groups were conventional brackets (Mystique; Dentsply GAC; and Clarity; 3M Unitek, Monrovia, Calif). The brackets were randomly bonded to the canines, first and second premolars, and first and second molars in the mandibular left hemiarch of 10 male participants. Biofilm was collected from the tooth surfaces before bonding and from the brackets on day 21 and placed in Petri dishes containing Mitis salivarius agar. The brackets were removed on day 28 and examined by using scanning electron microscopy. Statistical analysis, analysis of variance, and the Tukey correction with a P value of 0.05 were used.
Results
The greatest numbers of colonies were found in an active self-ligating bracket group (In-Ovation C), and the fewest colonies were in a conventional bracket group (Clarity). The largest colonies formed on active self-ligating brackets. In the slot, the greatest formation was in a control group (Mystique).
Conclusions
Self-ligating esthetic brackets do not promote greater or lesser S mutans colonization when compared with conventional brackets. Differences were found to be related to the material composition of the bracket.
The diversity of devices used in orthodontic appliances can promote specific alterations in the oral environment, such as acidic pH, greater adherence of microorganisms ( Streptococcus mutans ), and the development of biofilm. These alterations increase the risk of enamel decalcification. Clinical characteristics and physical properties of brackets vary considerably, and these can directly influence the adherence of dental plaque and consequently cause gingivitis. A direct relationship exists between gingival inflammation and dental plaque: with more gingivitis, there is greater colonization of bacterial plaque. The surface characteristics of the teeth and gingivae, and salivary secretions can influence the quantity and quality of biofilm formation. The porous structure of the material of the brackets provides a highly favorable ecologic niche for the adherence of microorganisms and the continuous development of biofilm.
Self-ligating brackets have a mechanical mobile device to close the slot, converting it into a tube. Some systems are considered passive (Damon 3, Smart Clip, Vision, Oyster), and others are active (Speed, In-Ovation, QuicKlear). Some advantages are attributed to the self-ligating bracket systems: eg, more rapid orthodontic movement and consequent reduction in treatment time for the mechanical closing of spaces. However, it is not known whether adherence of microorganisms and the development of biofilm are diminished when self-ligating brackets are used, because the ligatures (metallic or elastic) necessary for maintaining the orthodontic wire in place with conventional brackets are replaced by the opening and closing mechanism (clip) of the self-ligating brackets. The alteration of microbial adherence depends also on factors such as variations in design, size, and composition of the self-ligating and conventional esthetic brackets, methods of bonding and of tying the wire to the slot, level of oral hygiene, and age of the patient.
As the numbers of adult patients grow, so does the demand for esthetic self-ligating bracket systems. Thus, the aim of this study was to evaluate whether self-ligating esthetic brackets have advantages over conventional esthetic brackets with respect to surface retention of S mutans colonies.
Material and methods
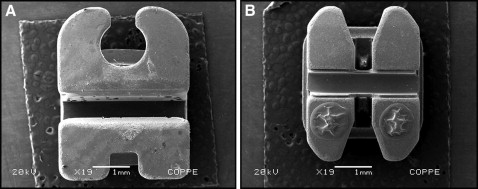
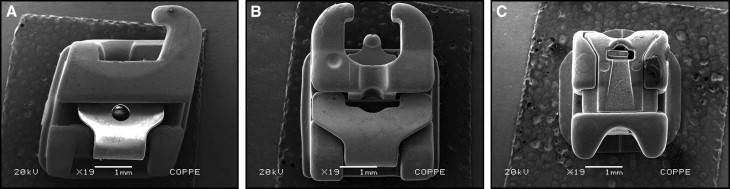
The sample consisted of 50 esthetic brackets—20 conventional and 30 self-ligating—bonded in 10 male volunteers (ages, 28-40 years). One week after receiving oral hygiene instructions, 1 bracket of each type was placed in each subject. The conventional brackets were Mystique (Dentsply GAC, Bohemia, NY) ( Fig 1 , A ) and Clarity (3M Unitek, Monrovia, Calif) ( Fig 1 , B ). Two of the self-ligating brackets were active types: active QuicKlear (Forestadent, Pforzheim, Germany) ( Fig 2 , A ) and In-Ovation C (Dentsply GAC) ( Fig 2 , B ); the other self-ligating bracket was passive: Damon 3 (Ormco, Glendora, Calif) ( Fig 2 , C ). The conventional brackets, with elastic ties, were used as controls, and the self-ligating brackets were the experimental units. The 10 volunteers were randomly selected and had complete permanent dentitions. Exclusion criteria included orthodontic treatment, carious lesions, periodontal problems, and antimicrobial use in the last 3 months. This study was approved by the ethics committee of Universidade do Estado do Piauí (UESPI) (protocol number 28625). The subjects received basic oral hygiene instructions, with the intent to standardize brushing during the study. They were taught the modified Bass technique and were given an oral hygiene kit that included a toothbrush (Procter & Gamble/Oral B, São Paulo, Brazil) and toothpaste (Colgate-Palmolive, São Paulo, Brazil).
The first biofilm samples were collected before bonding. The patients were instructed not to eat food and not to brush their teeth for a minimum of 12 hours before the collection of the dental biofilm. Plaque was collected with size 20 absorbent paper cones (Dentsply Indústria e Comércio, Petrópolis, Rio de Janeiro, Brazil), obtained from the mandibular canines, first and second premolars, and first and second molars from the supragingival areas: cervical-buccal, mesial-interproximal, and distal-interproximal surfaces.
The experiment then proceeded with the bonding of 5 different brackets in each patient. The brackets were randomly assigned to selected teeth: canines, first and second premolars, and first and second molars of the mandibular left hemiarch. All brackets were bonded with Transbond XT (3M Unitek), in a systematic manner and according the manufacturer’s instructions. No orthodontic wires were placed on any bracket; the conventional esthetic brackets received elastic ties, which are considered part of conventional brackets because of their function to retain the wire.
The second collection of biofilm was done 21 days after bonding the brackets and was obtained from the hooks, slots, and cervical regions. The material collected was placed in 1.5-mL sterile, plastic, single-use Eppendorf tubes (Axygen, Union City, Calif). These tubes were identified for each patient, tooth, and bracket, and weighed by precise electronic balance (model BG200; Indústria e Comércio Electro-electrônica Gehaka, São Paulo, São Paulo, Brazil). The quantity of biofilm collected was diluted and homogenized with a mechanical vibrator, by using 1 mL of saline solution composed of 0.85% sodium chloride and 1% of sodium thioglycolate for each 1 mg of plaque collected. With a micropipette, an aliquot of 0.1 mL of the suspended diluted medium was taken from the Eppendorf tube and placed in a test tube containing 0.9 mL of the same saline solution, and then the mixture was homogenized. Then 0.1-mL aliquots of each dilution were plated on Petri dishes containing Mitis salivarius culture medium selective for S mutans growth. The Petri dishes were incubated during the counting period (72 hours) at 38°C in anaerobic conditions. The selected plates had to show macroscopically visible colonies to accomplish the reading and counting of the colonies, which were performed by 1 previously trained and calibrated examiner (L.E.A.G.N.).
On day 28, the brackets were removed with orthodontic pliers (Rocky Mountain Orthodontics, Denver, Colo) and placed immediately in test tubes containing 0.5 mL of sterile saline solution (0.9% sodium chloride) to avoid harming the bacterial colonies. Later, the brackets were placed in wells that were numbered and identified by the patient and tooth to which the bracket belonged. They were submitted to fixation by a gradual series of alcohol concentrations of 50% to 70%, 75%, 90%, and 100%, each at a 10-minute interval. After this phase, all hydrous residues were eliminated by using a critical point dryer device (CPD 030; Bal-Tec AG, Balzers, Liechtenstein). The dehydrated brackets were prepared with a silver-based adhesive and underwent metallization with gold covering (Union FL 9496; Bal-Tec AG). The pieces were then placed on an acrylic plate and examined with a scanning electron microscope (SM 5310; JEOL, Tokyo, Japan).
Statistical analysis
The data were organized in tables, separated by types of brackets and by location of the collections from the brackets. The conventional brackets with elastic ties were used as controls, and the self-ligating brackets were the experimental units. The values were transformed by taking the root to obtain the normal and applying analysis of variance, and significant interactions between the groups were analyzed separately and together by using the Tukey method, with P = 0.05.
A distinction was made between brackets with the most and the least amounts S mutans colonies. The statistical analyses were repeated between the groups of brackets separately to determine whether there was a significant difference in the colonization of S mutans among them.
Results
Table I shows the mean values of bacterial plaque collected from dental surfaces before bonding the brackets.
| Area | Mean CFU |
|---|---|
| Cervical-buccal | 5.40 |
| Mesial-interproximal | 3.60 |
| Distal-interproximal | 3.40 |
Tables II through IV compile the data from the collection of microorganisms taken directly from the brackets (hook, slot, and cervical regions) used in the experiment. Table II gives the results of the evaluation of each bracket as a whole and shows that In-Ovation C had the highest numbers of colony forming units, and Clarity had the least. The largest colonies were formed on the active brackets. In Table III , it can be verified that the location on the bracket with the greatest formation of S mutans was in the slot, followed by the cervical region of the bracket, and then the hook. No statistically significant differences were observed between the conventional Mystique brackets and the self-ligating Damon 3, QuicKlear, and In-Ovation C brackets with respect to the numbers of colonies formed in the slots ( Table IV ). There was statistical similarity among the self-ligating brackets and the conventional Clarity brackets.
| Bracket | Mean total CFU | |
|---|---|---|
| Clarity | 508.47 | cd |
| Mystique | 679.60 | bc |
| Damon 3 | 705.07 | bc |
| QuicKlear | 909.87 | ab |
| In-Ovation C | 1043.80 | a |
| Location | Mean total CFU | |
|---|---|---|
| Hook | 51.67 | c |
| Slot | 1152.50 | a |
| Cervical | 908.87 | b |
| Bracket | Location | |||||
|---|---|---|---|---|---|---|
| Hook | Slot | Cervical | ||||
| Clarity | 7.20 | a B |
208.60 | c B |
1309.60 | ab A |
| Mystique | 6.00 | a C |
1427.20 | a A |
605.60 | cd B |
| Damon 3 | 84.60 | a B |
1190.00 | a A |
840.60 | bc A |
| QuicKlear | 110.60 | a C |
1620.40 | a A |
998.60 | abc B |
| In-Ovation C | 87.00 | a B |
1661.60 | a A |
1382.80 | a A |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


