Carbohydrates
Publisher Summary
In sheer amount, carbohydrates make up the bulk of organic matter on the earth. They are predominantly of plant origin, one of the most abundant forms being the structural carbohydrate cellulose. In higher animals, however, protein, rather than carbohydrate, is the principal structural material, although special types of carbohydrate, namely, the glycosaminoglycans, are important constituents of skeletal and other connective tissues. Other types of polysaccharide play a structural role in the cell walls of bacteria. These may act as antigens so that, when invaded, the body is able to defend itself by producing specific antibodies that lead to destruction of the bacteria. Apart from their structural importance, carbohydrates provide energy for synthetic processes and other work undertaken by cells and also provide many of the simple starting materials that are required for the synthesis of more complex substances. The carbohydrates of the diet are of special interest to the dental profession as their type and quality is an important factor affecting the health of the oral tissues. The chapter describes carbohydrate as a polyhydroxyaldehyde, polyhydroxyketone, or a substance that can be hydrolyzed to give these compounds. The carbohydrates are usually classified as monosaccharides, oligosaccharides, and polysaccharides.
1. Monosaccharides: simple sugars such as glucose, fructose and ribose which cannot be broken down into smaller units by mild hydrolysis.
2. Oligosaccharides: short chain compounds which on hydrolysis yield a limited number of monosaccharide units.
3. Polysaccharides: complex polymers which contain large numbers of monosaccharide units. They may be composed of only one type of monosaccharide, as in the case of starch, glycogen, cellulose and dextran, or of two or more different monosaccharides as in the glycosaminoglycans.
Monosaccharides
< ?xml:namespace prefix = "mml" />
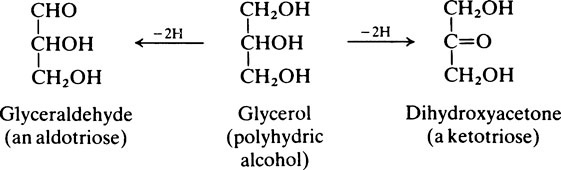
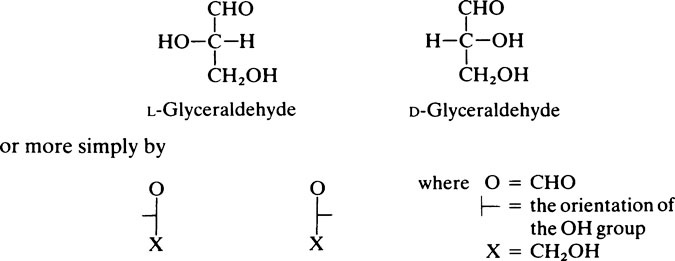
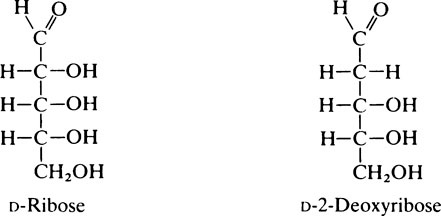
The naturally occurring sugars almost always belong to the D-series of compounds.
This proximity facilitates the formation of an oxygen-containing intramolecular ring which may be either a five-membered (C4 + O) furanose ring or a six-membered (C5 + O) pyranose ring. Compounds of this type which result from the condensation of an aldehyde group and a hydroxyl group are known as hemiacetals (page 10). Projection formulae such as the following, may be
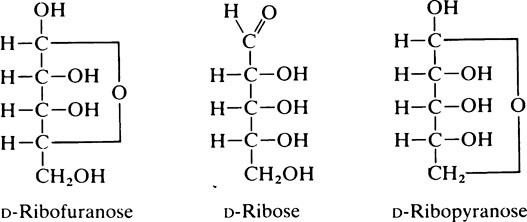
drawn showing these ring structures but they give a very distorted picture of the molecule and Haworth suggested that they should be replaced by perspective formulae. The perspective formulae for the pyranose and furanose forms of ribose are shown below with D-glucopyranose included for comparison:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses