Introduction
The aim of the present study was to evaluate DNA damage (micronucleus) and cellular death (pyknosis, karyolysis, and karyorrhexis) in exfoliated buccal mucosa cells from adults after fixed orthodontic therapy.
Material and Methods
A total of 23 healthy adults (10 men and 13 women) undergoing orthodontic therapy were included in this setting.
Results
The results pointed out no significant statistically differences ( P >0.05) of micronucleated oral mucosa cells. In the same way, orthodontic therapy was not able to increase other nuclear alterations closely related to cytotoxicity such as karyorrhexis, pyknosis and karyolysis ( P >0.05).
Conclusion
In summary, these data indicate that orthodontic therapy may not be a factor that induces chromosomal damage, nor it is able to promote cytotoxicity. Since DNA damage and cellular death are important events during carcinogenic processes, especially in early phases, this study represents a correct evaluation with respect to real health risks induced by orthodontic devices.
Environmental health sciences focus on the link between the presence of contaminants in the environment and their relation to possible adverse health effects. Within this context, human biomonitoring data have proved to be a valuable addition to, or have even surpassed, estimates of exposure based on environmental measures. Particularly, biomonitoring of human populations exposed to potential mutagens, cytotoxins, or even carcinogens can provide an early detection system for the initiation of cell deregulation in the development of cancer. As a result, several markers have been identified to monitor the exposure of humans to mutagens and carcinogens. Classically, mutagenicity tests evaluate chromosomal aberration and sister chromatid exchange. Data are accumulating that support the hypothesis that mutagenicity end points are predictors of human cancer risk. However, the relationship among DNA damage, persistence and repair, and mutagenic end points remains complex.
Intraoral fixed orthodontic appliances include brackets, bands, and arch wires that are made of alloys containing nickel, cobalt, and chromium in different percentages. Actually, different types of orthodontic brackets are available in the global market. The number of bracket systems for orthodontic therapy has increased significantly. In a previous study conducted by Faccioni et al , it was found that, as assessed by the single-cell gel (comet) assay in human patients, orthodontic therapy can induce DNA damage in oral mucosa cells as a result of nickel and cobalt released from fixed orthodontic appliances. The alkaline version of the single-cell gel (comet) assay is sensitive for a wide variety of DNA lesions. Among them are single strand and double strand breaks; oxidative DNA base damage; alkali-labile sites, including abasic and incomplete repair sites; and DNA-DNA/DNA-protein/DNA-drug cross-linking in any eukaryotic cell. However, the single-cell gel (comet) assay does not necessarily predict the mutagenic potential of metals; moreover, the genotoxicity can be modulated in combination with other DNA-damaging agents that are present in the environment. This is because the absence of a close relationship between DNA migration in the comet assay and mutagenesis may be explained by the fact that some effects seen in the comet assay occur as a consequence of an error-free DNA repair process. Herein, further evaluation of orthodontic therapy and mutagenicity and/or cytotoxicity is a necessary step to better establish the real health risks. For this purpose, a great deal of enthusiasm was raised by the application of the micronucleus test to uncultured exfoliated cells. Micronucleus arises from acentric fragments or whole chromosomes that are not included in the main nuclei of the daughter cells. The formation of micronuclei can be induced by substances that cause chromosome breakage (clastogens) as well as by agents that affect the spindle apparatus (aneugens). Recently, we have applied this methodology with success in individuals (adults and children) exposed to dental radiographs, or with malignant tumors undergoing radio- or chemotherapy. As a result and because of inappropriate in-vivo evidence, the present study was to investigate the frequency of micronucleated cells in oral mucosa from individuals who had submitted to fixed orthodontic therapy. To monitor cytotoxic effects, pyknosis, karyolysis and karyorrhexis were also evaluated in this setting. Certainly, such data will contribute to a better understanding of orthodontic therapy on the cellular system.
Material and methods
Subjects
The subjects of this study comprised a total of 23 healthy adults (10 men and 13 women) with a mean age of 18.5 ± 7 years who had submitted to orthodontic therapy at the Department of Orthodontics, São Paulo Methodist University, São Bernardo do Campo, Brazil. The fixed appliances consisted of an average of 4 to 8 bands and 20 bonded brackets. Brackets were provided by Abzil (São Paulo, Brazil): iron, 71%; nickel, 8%, and chromium, 19%. The arch wires used in this study were a nickel-titanium alloy (Morelli Dental Company, Sorocaba, Brazil): nickel, 50.8%; titanium, 49.2% during the treatment or stainless steel (Ormco Corporation, Orange, Calif): nickel, 8.6%; iron, 72.6%; chromium, 20% at the end of the orthodontic therapy.
Micronucleus test in oral mucosa cells
Exfoliated oral mucosa cells were collected before orthodontic therapy, during orthodontic therapy (defined as the time after the alignment and leveling with nickel titanium arches, ∼170 days after beginning the treatment), and after therapy (ie, at least 6 months after the insertion of stainless steel arches). After rinsing the mouth with tap water, cells were obtained by scraping the right/left cheek mucosa with a moist wooden spatula. Cells were transferred to a tube containing saline solution, centrifuged (800 rpm) over 5 minutes, fixed in 3:1 methanol/acetic acid, and dropped onto precleaned slides. Later, the air-dried slides were stained, using the Feulgen/Fast Green method, and examined under a light microscope at 400× magnification to determine the frequency of micronucleated cells as described elsewhere. Two thousand cells were scored from each patient for each sampling time (before, during, and at final orthodontic treatment).
Data analysis
Micronuclei were scored according to the criteria described by Sarto et al as a parameter for DNA damage (mutagenicity). For cytotoxicity, the following nuclear alterations were considered: pyknosis, karyolysis, and karyorrhexis. Results were expressed as a percentage together. Such analysis was established in a previous study conducted by our research group.
Statistical methods
The Friedman test for dependent samples was used to compare the frequencies of micronuclei and other cytotoxic alterations among the samples before, during, and at final orthodontic therapy. For this purpose, we used SigmaStat software, version 1.0 (Jandel Scientific, San Rafael, Calif). The level of statistical significance was set at 5%.
Results
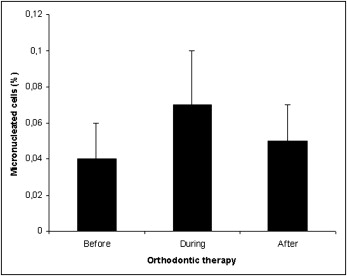
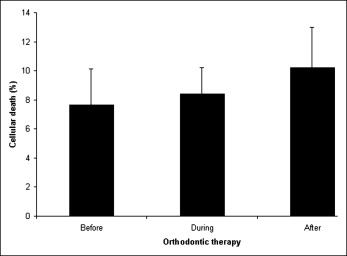
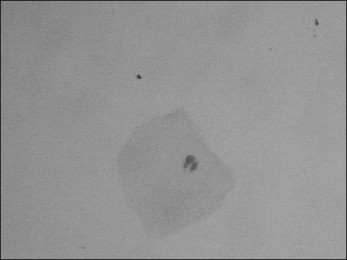
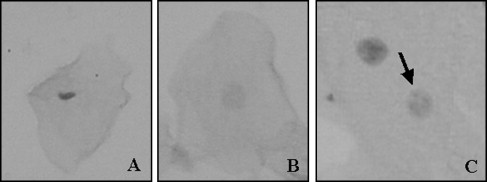
Figure 1 shows the frequencies of micronucleated cells in individuals undergoing orthodontic therapy. Before the beginning of orthodontic device exposure, the mean frequency of micronucleated cells was 0.04%. No statistically significant differences ( P >0.05) were noticed either during or after orthodontic therapy. In the same way, an increase of other nuclear alterations were not observed throughout the experimental design, as depicted by the frequency of karyorrhexis, pyknosis, and karyolysis. These data are summarized in Figure 2 . Figure 3 shows a micronucleated cell, and Figure 4 displays abnormalities closely related to cytotoxicity, ie, karyorrhexis, pyknosis, and karyolysis.
To avoid putative confounding factors, all participants of this study were nonsmokers. In addition, exposure to known genotoxins was not related to any of the study participants. A total of 3 individuals included in this trial used oral antiseptic solutions (chlorhexidine, Listerine) regularly. Daily alcohol consumption was not considered in this study because a recall bias phenomenon has occurred.
Results
Figure 1 shows the frequencies of micronucleated cells in individuals undergoing orthodontic therapy. Before the beginning of orthodontic device exposure, the mean frequency of micronucleated cells was 0.04%. No statistically significant differences ( P >0.05) were noticed either during or after orthodontic therapy. In the same way, an increase of other nuclear alterations were not observed throughout the experimental design, as depicted by the frequency of karyorrhexis, pyknosis, and karyolysis. These data are summarized in Figure 2 . Figure 3 shows a micronucleated cell, and Figure 4 displays abnormalities closely related to cytotoxicity, ie, karyorrhexis, pyknosis, and karyolysis.




