One of the primary reasons for the development of base metal alloys for dental applications has been the escalating cost of gold throughout the 20th century. In addition to providing lower cost alternatives, these nonprecious alloys were also found to provide better mechanical properties and aesthetics for some oral applications. Additionally, certain base metal alloy systems are preferred because of their superior mechanical properties, lower density, and in some cases, their capability to osseo-integrate. The base metal alloy systems most commonly used in dentistry today include stainless steels, nickel-chromium, cobalt-chromium, titanium, and nickel-titanium alloys. Combined, these alloy systems provide a wide range of available properties to choose the correct material for both temporary and long-term restoration and implant applications.
The history of dental restorations and implants dates back to the ancient Egyptians, who used sea shells on corpses for burial, and the Etruscans, who used bone and bands of gold wire to replace missing teeth . The modern era of dental restorations began just after the turn of the 20th century with the use of a number of precious metals, as well as some attempts to use zinc, steel, copper, and even brass . The success of some of these materials as well as fluctuations in the cost and availability of gold, which exploded to nearly $900 an ounce in 1980, have been credited with driving dentistry toward the development and use of alternative metal alloys . During the 20th century, the efforts of numerous researchers have lead to the development of new alloys that are not only less expensive than gold, but have properties more suitable for specific applications. Many of the modern alloy systems do not include precious metals as previously used in dentistry. Because the primary alloying elements for these alloys do not include precious metals, they are referred to as base metal alloys. In this article, five base metals alloy systems used in dentistry—stainless steels, nickel-chrome alloys, cobalt-chromium alloys, titanium alloys, and super-elastic nickel-titanium alloys—are described in the order of their introduction into the field.
Stainless steel alloys
Stainless steels have been successfully used in orthopedics and dentistry for almost a full century. Sherman introduced vanadium surgical steel in 1912, when he found that it possessed elasticity and ductility not found in other steels; however, the vanadium steel had poor biocompatibility. In 1926, the 18-8—18% chromium (Cr)-8% nickel (Ni)—austenitic stainless steel was introduced because it was stronger and more corrosion-resistant in saline environments. Later that same year, Strauss patented a variation of this 18-8 steel that added 2% to 4% molybdenum (Mo) and made it even more resistant in acidic and chloride environments. The carbon content of this 18Cr-8Ni-Mo alloy was later lowered to 0.08%, and the alloy became known as 316 stainless steel. As is seen from the history of the development of austenitic stainless steel, the composition is based upon iron with the addition of chromium, nickel, and often molybdenum as the primary alloying elements. Chromium and molybdenum are added for corrosion resistance and significant amounts of nickel are added to stabilize the austenitic structure. Additional common alloying elements include silicon, manganese, niobium, titanium, and carbon. Although austenitic steels are not hardenable by heat treatments, the large amount of ductility allows them to undergo substantial cold working before fracture . The major austenitic stainless steels used in dentistry today are still the same 18-8 (302 and 304) and 316 alloy.
Since its introduction in the form of hand-drawn archwire around 1929, 18-8 stainless steel has been extensively used in dentistry . During the 1960s, stainless steel orthodontic wires were overtaking gold because of the higher stiffness, and the smaller, more esthetic, size of the restoration . Because of their low costs, good mechanical properties, and adequate corrosion resistance in the oral environment, austenitic stainless steels are commonly used today for orthodontic wire and appliances, temporary crowns, magnetic connectors and clips, and dental implants .
As shown in Table 1 , the 18-8 stainless steel orthodontic archwires are considerably stronger than the cobalt-chromium nickel (Co-Cr-Ni), titanium-molybdenum (Ti-Mo), and nickel-titanium (Ni-Ti) wires. The manufacturing process of stainless steel wires cold-works the material, which reduces its ductility. A recovery heat treatment process between 400°C and 500°C will relieve such residual stresses in the material from manufacturing and thus improve its elastic properties . Several additional mechanical property terms used in classifying orthodontic wire are outside of those normally described in material property characterization. Formability has been defined as the ability to bend the wire into the configuration desired by the clinician ; 18-8 stainless steel wires have been shown to have excellent formability . Stiffness is proportional to the elastic modulus, and is a measure of the force applied by the restoration to the teeth . As shown in Table 1 , the elastic modulus of stainless steel is approximately twice that of gold, but lower than the Co-Cr-Ni wires, and therefore stainless steel wires have a stiffness value in between the gold and Co-Cr-Ni wires. Resiliency reflects the work ability of the wire acting as a spring . Stainless steel wires have a favorable resiliency compared with gold wires, and have been shown to be able to do the work needed clinically to move teeth . Flexibility or springback is defined as the materials maximum elastic deflection, and is the ratio of the yield strength over the modulus of elasticity . The springback of stainless steel wires has been shown to be slightly higher than that of gold wires, but lower than most Ti-Mo and Ni-Ti wires . Finally, the joinability of a wire is the ability to weld or solder to attachments. Soldering of the stainless steel wires is possible, but can be somewhat difficult .
| Alloy | Condition | Tensile strength (MPa) | Yield strength (MPa) | Elastic modulus (GPa) | Elongation (%) | Density (g/cm 3 ) |
|---|---|---|---|---|---|---|
| Stainless steel | ||||||
| 18-8 wire | As received | 2035–2849 | 965–1680 | 134–200 | 2–3.2 | 8 |
| Stress relieved | 2160 | 1034–1950 | 134–200 | — | 8 | |
| 316 wire | As received | 2275–2351 | 1955–2070 | 185–191 | — | — |
| 316L | Annealed | 550–600 | 220–331 | 190–200 | 50–55 | 7.95 |
| Cold-worked | 896–1014 | 790–827 | 167–200 | 20–25 | 7.9–7.95 | |
| 108 | Annealed | 931 | 586 | 188 | 52 | 7.63 |
| Cold-worked | 1344 | 1179 | 188 | 35 | 7.63 | |
| Nickel-Chromium | ||||||
| Ni-Cr-Be | Casting | 778–1355 | 325–838 | 165–210 | 3–23.9 | 7.9–8.1 |
| Ni-Cr | Casting | 539–919 | 180–858 | 141–248 | <1–32.6 | 7.9–8.7 |
| Cobalt-Chromium | ||||||
| Co-Cr-Mo | Casting | 655–889 | 390–644 | 155–240 | 1.5–10 | 8.5 |
| Co-Ni-Cr-Mo | Wrought/annealed | 795–1007 | 240–655 | 232 | 50–70 | 9.2 |
| Co-Cr-Ni | Casting | 685 | 470 | 198 | 8.0 | 7.5–8.5 |
| Co-Cr-Ni wire | As received | — | 827–1241 | 146–198 | — | 7.5–8.5 |
| Stress relieved | — | 1103–1378 | 179–204 | 8.0 | 7.5–8.5 | |
| Titanium | ||||||
| CP Ti | Casting | 240–550 | 170–480 | 96–114 | 7.9–20 | 4.4–4.5 |
| CP Ti—grade 1 | Annealed | 240 | 170 | 100–103 | 24 | 4.51 |
| CP Ti—grade 2 | Annealed | 345 | 275 | 100–103 | 20 | 4.51 |
| CP Ti—grade 3 | Annealed | 450 | 380 | 100–103 | 18 | 4.51 |
| CP Ti—grade 4 | Annealed | 550 | 485 | 100–104 | 15 | 4.51 |
| CP Ti—grade 4 | Cold-worked | 760–888 | 485–725 | 110 | — | 4.51 |
| Ti-6Al-4V ELI | Annealed | 860–1076 | 520–896 | 110–116 | 10–15 | 4.43–4.5 |
| Ti-6Al-4V | Casting | 877–930 | 830–870 | 113–137 | 2.1–12 | — |
| Ti-6Al-7Nb | Annealed | 978–1024 | 913 | 105 | — | 4.52 |
| Ti-15Mo | Annealed/aged | 874 | 544 | 78 | — | 4.96 |
| TMA wire | As drawn | — | 621–1172 | 60–69.6 | — | — |
| Heat-treated/aged | — | 1220–1390 | 92.4–95.1 | — | — | |
| Nickel-Titanium | — | — | — | — | — | |
| Ni-Ti wire-A | 527–1380 | 230–379 | 120 | 13–40 | 6.45 | |
| Ni-Ti wire-M | — | 207–552 | 32–50 | — | — | |
| Other | — | — | — | — | — | |
| Pure gold | 130 | 20 | 90 | 45 | 19.3 | |
| Type III and IV | — | — | — | — | — | |
| Gold casting | — | 207–434 | 90 | 10–39 | 11.3–15.5 | |
| Cortical bone | 100–200 | 130 | 10–20 | 1–3 | 0.7 (dry) | |
| Cancellous bone | 10–20 | — | 0.2–0.5 | 5–7 | — | |
In general, the 18-8 stainless steel orthodontic wires have shown at least adequate corrosion resistance in the oral environment. Kim and Johnson performed a potentiodynamic comparative study of 18-8 stainless steel, Ti-Mo, and Ni-Ti orthodontic wires in a 0.9% sodium chloride (NaCl) solution, and recorded an oxide film breakdown potential (E br ) of 400 mV for the stainless steel. This E br value was inside the range of those reported for Ni-Ti, but significantly less than those for the Ti-Mo wire. The stainless steel wire corroded readily in the saline solution and revealed more surface pitting when compared with the Ni-Ti wires, indicating that the stainless steel is more susceptible to corrosion; however, Sarkar and colleagues performed a similar cyclic polarization study of the common types of orthodontic wires in a 1% NaCl solution and found no oxide breakdown in the stainless steel orthodontic wire, whereas the Ni-Ti wire showed oxide layer breakdown and extensive pitting corrosion. In this study, the potentials were only driven to +300 millivolts (mV) versus standard calomel electrode (SCE), and the Ni-Ti wire still experienced oxide breakdown at a potential slightly over +100 mV versus SCE. In addition, Lin and colleagues showed that the brand of commercially available 18-8 stainless steel orthodontic bracket, and thus the processing techniques, showed more of an effect on the corrosion resistance of the appliance than differences in initial surface roughness or manufacturing defects. Shin and colleagues found similar results in an immersion study on stainless steel orthodontic archwires, stress relieved at 500°C, in artificial saliva. This study concluded that uniform surface corrosion was detected on the heat-treated arch wires after a 12-week immersion in artificial saliva. Crevice corrosion was also observed between the brackets and banding material. The corrosion resistance of stainless steels with higher carbon contents may be decreased during heat treatments between 400°C and 900°C, through the formation of chromium-iron carbides at the grain boundaries. These chromium-iron carbides result in a localized chromium depletion in the oxide layer, thus creating intergranular corrosion . Therefore, the corrosion shown by Shin and colleagues may have been caused by the formation of chromium-iron carbides at the grain boundaries. Kerosuo and colleagues found significantly greater releases of nickel from a fixed stainless steel orthodontic appliance under dynamic conditions simulating more realistic in vivo applications, as compared with static dissolution. Chromium release was also detected from this appliance, but was not significantly different comparing static and dynamic dissolution conditions . Nonetheless, Staffolani and colleagues determined daily Ni and Cr release levels from orthodontic appliances in acid solutions to be well below the levels ingested in the daily diet.
In addition to orthodontic wire applications, 18-8 stainless steels have also been used for adolescent and temporary adult preformed metal crowns for more than 50 years . In addition to low costs, stainless steel crowns offer good corrosion resistance, ductility, and the ability to work-harden during the crimping process. Some clinicians have suggested these preformed stainless crowns to be easier to place in certain difficult patients, such as a crying child, when compared with amalgams ; however, other practitioners suggest that these restorations are too time-consuming to fit, inappropriate for many applications, difficult to manipulate, and not aesthetically pleasing in the oral cavity .
Some manufacturers have elected to use a more corrosion-resistant 316 steel in place of the more prevalent 302/304 stainless steel used in orthodontics. Peterson recommended the 316 alloy shortly after WWII as the steel with the best combination of properties for bone plates and screws used for fracture fixation treatments by the Army . Some time after that, it was introduced as a new orthodontic wire in dentistry. The addition of 2% to 4% Mo to 316 gave this new orthodontic wire superior corrosion resistance in addition to its superior elastic properties, compared with 18-8 steels . Further studies on orthodontic brackets and molar bands have suggested reduced staining of the enamel and metal ion release in 316 compared with 18-8 orthodontic brackets and bands .
During the 1950s, the carbon content of 316 was lowered from 0.08% to 0.03%, and an ‘L” (low carbon) was added to the designation (316L). In the 1960s and 1970s, implant retrieval studies and research suggested the need to make further changes in the composition requirements of implant- quality 316L stainless steel (American Society of Testing Materials [ASTM] F138 ) by adjusting the limits of Ni, Cr, and Mo . The ASTM F138 standard uses a compositional pitting resistance equivalent (PRE) function to insure that the Cr and Mo levels are adequate to control pitting corrosion. The tighter restrictions on Cr and Mo levels under ASTM F138 make 316L more corrosion-resistant than the 18-8 and 316 steels commonly used in dentistry. This additional corrosion resistance translates to lower metal ion release levels into the surrounding tissues, and thus less chance of an inflammatory reaction by the patient and less device loosening over time . Several studies have characterized the mechanical , torsional , notched sensitivity , corrosion , stress corrosion cracking , and smooth and notched corrosion fatigue properties of implant quality 316L, and compared them with other austenitic stainless steels used for implants. Because most implant failures in vivo are caused by fatigue, the corrosion fatigue performance of these materials in saline environments at physiological temperatures is of particular interest. Showing a lower smooth sample fatigue strength compared with other austenitic steels used in orthopedics, 316L demonstrated the lowest fatigue notch sensitivity of the group. Therefore, the presence of a defect or scratch in the device should not substantially accelerate the failure of a 316L appliance or implant in vivo.
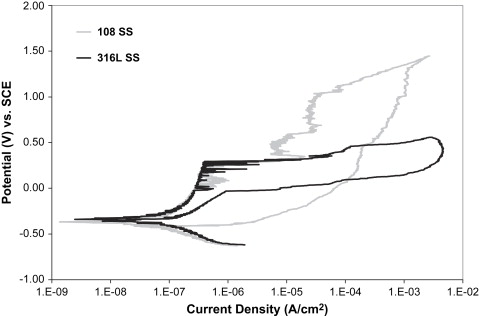
Nickel is the most hypersensitive metal, and is a major component of most austenitic stainless steels. Although 10% to 20% of the population is hypersensitive to nickel, relatively few of these sensitive individuals have experienced metal sensitivity reactions caused by implants . Nevertheless, the potential accumulation of nickel from the release over the lifetime of a restoration or implant remains a concern for patient hypersensitivity reactions. In part as a response to concerns over such sensitivity reactions, low-nickel (≤0.05%) austenitic stainless steels have recently been developed, providing a “nickel-free” alternative and presenting some interesting mechanical and corrosion properties. Instead of adding nickel, these alloys use a high nitrogen content (>0.90%) to stabilize the austenitic microstructure. This high nitrogen content also strengthens the alloy and contributes to its corrosion resistance . Corrosion studies on one such alloy, BioDur108 (108) (Carpenter Technology, Wyomissing, Pennsylvania), in the laboratories of the author’s institution revealed a large passive range, with an E br value that is far superior to 316L, as shown in Fig. 1 . In the same austenitic steel studies mentioned above, 30% cold-worked 108 showed the highest smooth and notched sample fatigue strengths. In addition, this nitrogen-strengthened austenitic steel retained substantial ductility after 30% cold working (see Table 1 ). The strength of annealed 108 is similar to cold-worked 316L, and the ductility of 108 is far superior (see Table 1 ). Fatigue studies on 108 in the annealed condition are currently under way in the author’s institution’s laboratories. The combination of superior ductility, fatigue strengths, and low levels of nickel (<0.05%) in the composition make this alloy worth consideration for future dental use .
Nickel-chromium alloys
Because of the lower cost of nickel compared with gold, starting in the 1930s, Ni-Cr castings such as Lunorium (Salabes Research Laboratories, Inc., Baltimore, Maryland) , Ticonium (Albany, New York) , and those meeting the composition requirements under the Touceda patent were introduced for crowns, bridges, and partial denture frameworks. In addition to the financial benefits, the Ni-Cr alloys have superior properties for use in porcelain-fused-to-metal (PFM) applications. These superior properties include a higher hardness values and substantially higher modulus of elasticity when compared with gold, as shown in Table 1 . This increase in the modulus of elasticity allows the cross-sectional thickness of the restoration to be decreased, and allows more space for the porcelain veneer, while still providing the appropriate strengths . Also, the thermal coefficients of expansion of the Ni-Cr alloys is closer to the porcelain veneers, which helps prevent cracking during heating and cooling cycles encountered by the restoration .
Ni-Cr alloys are generally divided into groupings based on the chromium, molybdenum, and beryllium (Be) content in the bulk composition. A number of researchers have separated the alloys into two or three compositional groupings with some variations in the exact criteria. A combination of the divisional criteria of some of these researchers yields three alloy classes: (1) Ni High-Cr (16%–27% Cr) High-Mo (>6% Mo), (2) Ni-Cr, and (3) Ni-Cr-Be (beryllium added) alloys . Be is added to enhance castability by lowering the melting range of the alloy and also as a grain refiner . O’Connor and colleagues found Ni-Cr-Be alloys to cast almost twice the number of segments as the next alloy grouping, in a large-scale dental alloy casting study that included Ni-Cr-Be, Ni-Cr, and Co-Cr as well as noble and precious alloys systems. In addition, the same study showed that the beryllium-containing Ni-Cr alloys produced better porcelain-metal bonds compared with the Ni-Cr alloys without beryllium. Molybdenum, titanium, and manganese additions all increase corrosion resistance . Molybdenum is also added to decrease the thermal expansion coefficient . Aluminum additions increase strength and hardness . In partial denture frameworks, carbon is added for enhanced yield strength and hardness, but it reduces ductility . The numerous alloying elements available in the Ni-Cr alloys present a wide range of microstructures with solid solution matrixes and intermetallic compounds. The heterogeneous surfaces and oxide layers contribute to a wide range of corrosion resistance, because of preferential ion release from certain areas on the surface, which may lead to internal galvanic coupling.
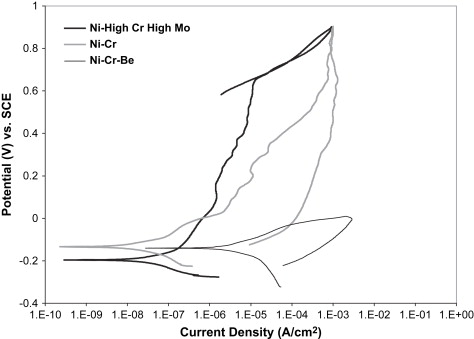
The wide range of corrosion response in Ni-Cr dental alloys allows the three compositional groupings mentioned earlier to also be separated by their corrosion resistance to acidic or chloride-containing solutions. Fig. 2 provides representative cyclic polarization scans from unpublished research data comparing the three classes of Ni-Cr alloys in a lactic acid-based artificial saliva solution at pH 4.6. The representative Ni High-Cr High-Mo alloy provided the lowest corrosion rate, as represented by the low corrosion current density (I corr ) value, and the highest breakdown or pitting potential (E br ) of the three alloy classes, which translates to a lower amount of metal ion release, and thus less chance of an allergic reaction by the patient. In cyclic polarization tests, the area of the hysteresis between the forward and reverse voltage scans is indicative of the amount of pitting corrosion taking place. Pitting corrosion changes the area and possibly the composition of the surface and oxide layers, and thus changes the alloy response, which creates an area mismatch between the forward and reverse scans. The Ni High-Cr High-Mo alloy revealed no hysteresis between the forward and reverse scans, indicating a lack of pitting corrosion. The Ni-Cr alloy showed a similar corrosion rate, but a substantially lower E br value, and a hysteresis between the forward and reverse scans, suggesting a less stable oxide and lower pitting resistance. Finally, the Ni-Cr-Be alloy revealed significantly higher I corr values, even lower E br values, and a large hysteresis, indicating a substantially significant increase in pitting corrosion rate and thus metal ion release into the surrounding tissues, compared with the other Ni-Cr alloys. Several studies have shown that the release of metal ions from all classes of Ni-Cr casting alloys is not proportional with the bulk alloy composition . Metal ion release from Ni-Cr alloys is normally mostly nickel, but the alloying element release can vary substantially because of selective leaching. Acidic environments can substantially increase the amount of nickel released. Wataha and colleagues reported more nickel release in 30 minutes in an acid environment than a full year in a neutral environment. In Ni-Cr-Be alloys, Be ions are released at an accelerated rate, because of the Ni-Be eutectic phase that is selectively attacked on the surface of the alloy. Bumgardner and Lucas found that Be ions were released initially at four to six times the bulk Ni-Cr-Be alloy composition in a cell culture dissolution study in 5% fetal bovine serum. Tai and colleagues , also studying a Ni-Cr-Be alloy system, concluded that occlusal wear may further increase metal ion release of nickel and beryllium to two or three times the levels from dissolution alone.
It should also be noted that the mechanical and corrosion properties of these alloys may be significantly affected by the heat treatments used during the PFM firing cycle. Winkler and colleagues and Marinello and colleagues reported decreases in hardness, and Morris found a significant reduction in strength on Ni-based alloys, after the PFM firing cycle. Roach and colleagues showed a substantial increase in corrosion rates for the Ni High-Cr High-Mo class and some of the Ni-Cr alloy class as a result of heat treatments used in porcelain firing, whereas Ni-Cr-Be alloys were not significantly affected. An important side note from this study was that the oxide breakdown potentials of the alloys were not significantly affected by the PFM firing processes, which agreed with studies by De Micheli and Meyer .
Because between 10% and 20% for the population is hypersensitive to nickel, the release of metal ions from Ni-based restorations into the surrounding tissues is a primary concern . In general, women also demonstrate a higher sensitivity to nickel than men, in part because of sensitization caused by jewelry containing nickel . Despite the hypersensitivity of some patients to nickel, Jones and colleagues concluded that a history of nickel allergy does not necessarily prevent a patient from successfully wearing a nickel-containing prosthesis.
Nickel-chromium alloys
Because of the lower cost of nickel compared with gold, starting in the 1930s, Ni-Cr castings such as Lunorium (Salabes Research Laboratories, Inc., Baltimore, Maryland) , Ticonium (Albany, New York) , and those meeting the composition requirements under the Touceda patent were introduced for crowns, bridges, and partial denture frameworks. In addition to the financial benefits, the Ni-Cr alloys have superior properties for use in porcelain-fused-to-metal (PFM) applications. These superior properties include a higher hardness values and substantially higher modulus of elasticity when compared with gold, as shown in Table 1 . This increase in the modulus of elasticity allows the cross-sectional thickness of the restoration to be decreased, and allows more space for the porcelain veneer, while still providing the appropriate strengths . Also, the thermal coefficients of expansion of the Ni-Cr alloys is closer to the porcelain veneers, which helps prevent cracking during heating and cooling cycles encountered by the restoration .
Ni-Cr alloys are generally divided into groupings based on the chromium, molybdenum, and beryllium (Be) content in the bulk composition. A number of researchers have separated the alloys into two or three compositional groupings with some variations in the exact criteria. A combination of the divisional criteria of some of these researchers yields three alloy classes: (1) Ni High-Cr (16%–27% Cr) High-Mo (>6% Mo), (2) Ni-Cr, and (3) Ni-Cr-Be (beryllium added) alloys . Be is added to enhance castability by lowering the melting range of the alloy and also as a grain refiner . O’Connor and colleagues found Ni-Cr-Be alloys to cast almost twice the number of segments as the next alloy grouping, in a large-scale dental alloy casting study that included Ni-Cr-Be, Ni-Cr, and Co-Cr as well as noble and precious alloys systems. In addition, the same study showed that the beryllium-containing Ni-Cr alloys produced better porcelain-metal bonds compared with the Ni-Cr alloys without beryllium. Molybdenum, titanium, and manganese additions all increase corrosion resistance . Molybdenum is also added to decrease the thermal expansion coefficient . Aluminum additions increase strength and hardness . In partial denture frameworks, carbon is added for enhanced yield strength and hardness, but it reduces ductility . The numerous alloying elements available in the Ni-Cr alloys present a wide range of microstructures with solid solution matrixes and intermetallic compounds. The heterogeneous surfaces and oxide layers contribute to a wide range of corrosion resistance, because of preferential ion release from certain areas on the surface, which may lead to internal galvanic coupling.
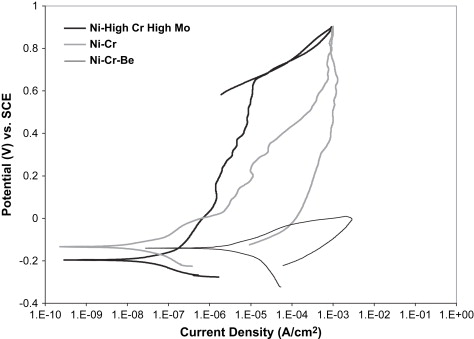
The wide range of corrosion response in Ni-Cr dental alloys allows the three compositional groupings mentioned earlier to also be separated by their corrosion resistance to acidic or chloride-containing solutions. Fig. 2 provides representative cyclic polarization scans from unpublished research data comparing the three classes of Ni-Cr alloys in a lactic acid-based artificial saliva solution at pH 4.6. The representative Ni High-Cr High-Mo alloy provided the lowest corrosion rate, as represented by the low corrosion current density (I corr ) value, and the highest breakdown or pitting potential (E br ) of the three alloy classes, which translates to a lower amount of metal ion release, and thus less chance of an allergic reaction by the patient. In cyclic polarization tests, the area of the hysteresis between the forward and reverse voltage scans is indicative of the amount of pitting corrosion taking place. Pitting corrosion changes the area and possibly the composition of the surface and oxide layers, and thus changes the alloy response, which creates an area mismatch between the forward and reverse scans. The Ni High-Cr High-Mo alloy revealed no hysteresis between the forward and reverse scans, indicating a lack of pitting corrosion. The Ni-Cr alloy showed a similar corrosion rate, but a substantially lower E br value, and a hysteresis between the forward and reverse scans, suggesting a less stable oxide and lower pitting resistance. Finally, the Ni-Cr-Be alloy revealed significantly higher I corr values, even lower E br values, and a large hysteresis, indicating a substantially significant increase in pitting corrosion rate and thus metal ion release into the surrounding tissues, compared with the other Ni-Cr alloys. Several studies have shown that the release of metal ions from all classes of Ni-Cr casting alloys is not proportional with the bulk alloy composition . Metal ion release from Ni-Cr alloys is normally mostly nickel, but the alloying element release can vary substantially because of selective leaching. Acidic environments can substantially increase the amount of nickel released. Wataha and colleagues reported more nickel release in 30 minutes in an acid environment than a full year in a neutral environment. In Ni-Cr-Be alloys, Be ions are released at an accelerated rate, because of the Ni-Be eutectic phase that is selectively attacked on the surface of the alloy. Bumgardner and Lucas found that Be ions were released initially at four to six times the bulk Ni-Cr-Be alloy composition in a cell culture dissolution study in 5% fetal bovine serum. Tai and colleagues , also studying a Ni-Cr-Be alloy system, concluded that occlusal wear may further increase metal ion release of nickel and beryllium to two or three times the levels from dissolution alone.
It should also be noted that the mechanical and corrosion properties of these alloys may be significantly affected by the heat treatments used during the PFM firing cycle. Winkler and colleagues and Marinello and colleagues reported decreases in hardness, and Morris found a significant reduction in strength on Ni-based alloys, after the PFM firing cycle. Roach and colleagues showed a substantial increase in corrosion rates for the Ni High-Cr High-Mo class and some of the Ni-Cr alloy class as a result of heat treatments used in porcelain firing, whereas Ni-Cr-Be alloys were not significantly affected. An important side note from this study was that the oxide breakdown potentials of the alloys were not significantly affected by the PFM firing processes, which agreed with studies by De Micheli and Meyer .
Because between 10% and 20% for the population is hypersensitive to nickel, the release of metal ions from Ni-based restorations into the surrounding tissues is a primary concern . In general, women also demonstrate a higher sensitivity to nickel than men, in part because of sensitization caused by jewelry containing nickel . Despite the hypersensitivity of some patients to nickel, Jones and colleagues concluded that a history of nickel allergy does not necessarily prevent a patient from successfully wearing a nickel-containing prosthesis.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


