20
Alternatives to Conventional Techniques
In this chapter, we review four types of technique that can be used instead of the traditional methods discussed so far, although, as we will see, some of these methods yield poorer results. Nevertheless, they do have a series of advantages. The methods we review are the following:
- Jet injection.
- Electronic dental anesthesia.
- Computer‐controlled injection systems.
- Intranasal maxillary local anesthesia (Kovanaze®).
Jet Injection
This alternative injection technique is based on injecting the local anesthetic solution without a needle. The solution is propelled at speed in a fine, high‐pressure jet in such a way that it crosses the oral mucosa to reach the subcutaneous tissue painlessly; this is why it is also known as high‐pressure jet injection.
This technique arose from a workplace accident. Fuel oil under pressure accidentally penetrated the fingers of a diesel mechanic. The oil passed deep under the skin, leading to the loss of fingers owing to its toxic effect. However, the liquid did not hurt when it penetrated the skin (Figge and Scherer 1947; Stephens and Kramer 1964).
Marshall Lockhart is said to have patented the first high‐speed jet injection machine in 1936 (Warren et al. 1955; Bennett et al. 1971). However, it was first used in cadavers by Figge and Scherer (1947). In the same year, it was applied in clinical practice for the injection of procaine with epinephrine into the skin (Hingson and Hughes 1947). In 1958, Margetis used it for the first time to administer local dental anesthetic (Margetis et al. 1958). The device in all these cases was the Hypospray (Stephens and Kramer 1964; Schmidt 1966), which was first introduced in 1947 (Kutscher and Zegarelli 1965). Hypospray was followed by many other commercial devices (Annex 33), of which the most widely used today are Syrijet® and Injex®.
Distribution of the Solution
Distribution of the solution has been studied in cadavers (Figge and Scherer 1947; Kramer 1962; Whitehead and Young 1968), rats (Bennett et al. 1971; Ikehara et al. 1972; ElGeneidy et al. 1974), and dogs (Bell et al. 1971). Once discharged, the local anesthetic solution crosses the mucosa and tends to spread through the connective tissue laterally in parallel to the mucosa (Kramer 1962; Stephens and Kramer 1964; Bennett et al. 1971) and manages to cover 10–40 mm in 90% of cases (Whitehead and Young 1968). The amplitude also depends on the area of discharge (Garellek 1967), in such a way that it can reach 15 mm in the buccal, 10 mm in the palate, and only 5 mm in the mandibular sulcus.
The entry wound is round or slightly irregular, with a diameter similar to that of the needle as it enters tissue (Stephens and Kramer 1964). The solution enters the area of least resistance and therefore penetrates better in looser connective tissue (Bell et al. 1971; Bennett et al. 1971; ElGeneidy et al. 1974). However, it does not penetrate or does so slightly in barrier structures such as bone, periosteum, nerve stem, vessels, salivary glands, and muscle (Stephens and Kramer 1964; Kutscher and Zegarelli 1965; Bennett et al. 1971; Epstein 1971; ElGeneidy et al. 1974). When it penetrates the muscles, it does so across fascial planes but not within the muscle structure (Bennett et al. 1971).
Deep penetration depends on the following: (i) the discharge area – penetration is 5 mm in the buccal and 10–15 in the mandibular sulcus (Garellek 1967; Bennett et al. 1971 oral); and (ii) the quantity injected – penetration is 5 mm with 0.05 ml and 15 mm with 0.2 ml (Bennett et al. 1971).
Microlesions may remain after discharge (Stephens and Kramer 1964) with subcutaneous edema, separation of collagen fibers (Ikehara et al. 1972), and inflammatory reaction (ElGeneidy et al. 1974), which differ little from the microlesions caused by a needle. Furthermore, while the most concentrated anesthetic solutions (5% vs 2%) increase the frequency of tissue lesions (Bennett et al. 1971), they also enhance clinical outcomes (Lambrianidis et al. 1979–1980).
When we compare high‐pressure jet injection with conventional injection, we see that a needle makes it possible to place the anesthetic solution at the required depth and to administer the required quantity of solution at this level in such a way that it leaves a deposit (Stephens and Kramer 1964). However, with jet injection, we can see how it loses strength from the moment it crosses the mucosa and cannot reach very deep areas. In addition, the solution penetrates deeper, therefore a smaller quantity of anesthetic is administered (Stephens and Kramer 1964; Epstein 1971). Since it is injected suddenly, it mixes more with tissues without leaving a deposit from which it can then spread out (Bell et al. 1971). The microlesions produced by both systems are similar (Stephens and Kramer 1964; Ikehara et al. 1972; ElGeneidy et al. 1974).
Indications
As we have seen, this technique does not ensure deep anesthesia because the solution does not penetrate deeply into the tissue or, if it does, it delivers a small quantity of anesthetic. It can therefore prove useful in the following situations:
- As topical anesthetic it is very useful (Kutscher and Zegarelli 1965; Garellek 1967; Bennett and Monheim 1971; Epstein 1971; Boj 1992), especially in the palate (Stephens and Kramer 1964) since it can be injected using the conventional technique after a minute (Kutscher et al. 1964).
- For supra‐ and sub‐gingival anesthesia during scaling and root planning procedures, respectively. (Kutscher and Zegarelli 1965; Epstein 1971).
- Placement of rubber dam clamps, especially if subgingival clamp placement is necessary for appropriate isolation of the teeth (Garellek 1967; Epstein 1971; Boj 1992; Arapostathis et al. 2010).
- Simple surgical techniques such as extraction of mobile primary teeth (Saravia and Bush 1991; Boj 1992), lancing fluctuant abscesses (Garellek 1967; Epstein 1971), gingivectomy limited to one tooth (Epstein 1971), excision of small lesions such as papillomas and fibromas (Greenfield and Karpinski 1972), removal of bone spurs, etc.
- Placement and removal of fixed orthodontic devices that can irritate the gingival (Epstein 1971; Greenfield and Karpinski 1972), such as fixed space maintainers, bands, orthodontic arches and ligature ties, subgingival matrix bands, etc.
Disadvantages
The technique has various disadvantages owing to the characteristics of distribution of local anesthetic solution (see above):
- Insufficient depth of anesthesia to ensure pulpal anesthesia (Dabarakis et al. 2007): Björn used an electric pulp tester to show that pulpal anesthesia is achieved in 13% of cases (Lethinen 1979). Anesthesia of deeper tissues is also poorer, therefore it yields poorer results in surgical techniques, extractions (Kutscher and Zegarelli 1965; Saravia and Bush 1991; Boj 1992; Arapostathis et al. 2010), endodontics, pulpotomy (Epstein 1971; Boj 1992), crown cutting and bridges in vital teeth (Kutscher and Zegarelli 1965), obturations in permanent teeth (Epstein 1971; Saravia and Bush 1991; Boj 1992) and primary teeth – although there may be some success in the latter (Saravia and Bush 1991; Boj 1992; Grau et al. 1997; Miegimolle et al. 2005; Arapostathis et al. 2010) – and mandibular block (Stephens and Kramer 1964; Bennett and Monheim 1971; Boj 1992).
- Cost, since many of the devices used are very expensive.
Advantages
The advantages to this technique can be summarized as follows:
- The absence of a needle means could be a very positive attribute for those patients who fear needles, therefore it could prove very useful in children and fearful adults (Stephens 1962; Stephens and Kramer 1964; Greenfield and Karpinski 1972, Miegimolle et al. 2005).
- When this technique can be used, patients prefer it to conventional techniques in 75% of cases (Table 20.1).
- Injection is not painful in 85% of cases (Table 20.1).
- The risk of transmission of blood‐borne pathogens to the provider or dental assistant is reduced because there are no sharps or infectious waste.
Equipment
Annex 33 discusses the main devices used in dentistry. The main ones are Syrijet® (designed exclusively for dentistry) and Injex®, which is the smallest. We comment on both devices below.
Syrijet®
This device was introduced in 1971 (Annex 33) and is still in use (Figure 20.1). It can be purchased online. As it is specially designed for dentistry, it has no anesthetic solution deposit, but uses 1.8‐ml dental cartridges. The nozzle pressure is 2000 pounds per square inch (psi), which is lower than that of other devices designed to cross the skin (Stephens and Kramer 1964). In this case, it is applied to the oral mucosa with little or no damage. The solution can be administered gradually in doses of 0.05 ml, from a minimum of 0.05 ml to a maximum of 0.2 ml (Annex 33).
Table 20.1 Percentage of patients who do not feel pain, prefer the pressure jet injection technique, and have hematoma at the injection site in various clinical trials.
| Study data | Variables analyzed | ||||
|---|---|---|---|---|---|
| Reference | Device | Patients | No pain | Preference | Hematoma |
| Hingson and Hughes (1947) | Hypospray | Children/adults | 94% | — | — |
| Margetis et al. (1958) | Hypospray | Children/adults | 100% | — | 72% |
| Kutscher et al. (1964) | Hypospray | Children/adults | 100% | — | — |
| Kutscher and Zegarelli (1965) | Hypospray | Children/adults | — | — | 42% |
| Schmidt (1966) | Hypospray | Children | 95% | 81% | 10% |
| Whitehead and Young (1968) | Panjet | Children/adults | 84% | 78% | 81% |
| Bennett and Monheim (1971) | Syrijet® | Children/adults | — | 90% | 1.5% |
| Lambrianidis et al. (1979–1980) | Panjet | Children/adults | 98% | 98% | 10% |
| Saravia and Bush (1991) | Syrijet® | Children/adults | — | 73% | — |
| Grau et al. (1997) | Syrijet® | Children | 76% | — | 33% |
| Grau et al. (1997) | Syrijet® | Adults | 96% | — | 24% |
| Dabarakis et al. (2007) | Injex® | Adults | — | 18% | 15% |
| Arapostathis et al. (2010) | Injex® | Children | 30% | — | 61% |
| Average | 86% | 73% | 35% | ||
| Rounded average | 85% | 75% | 35% | ||

Figure 20.1 Syrijet®: high‐speed jet injection system.
The head of the device is contra‐angled to facilitate access to the different areas of the oral cavity. The nozzle of the jet has a rubber ring that can be adapted to the mucosa so that when the device is discharged, the rubber ring reduces the sensation of pressure (Epstein 1971). The ring is replaceable and can be sterilized. The problem with this device is its size (245 mm long) and weight (550 g) (Annex 33).
Injex®
Injex® first appeared in 1999 and has the advantage that it is small (only 75 g) (Annex 33) (Figure 20.2). It comes with a fixed ampoule reservoir (0.3 ml), but also has a transporter and adapter that makes it possible to load the reservoir with anesthetic solution in 1.8‐ml dental cartridges. The discharge is set at 0.15 ml, and the solution cannot be dosed. It is used only for anterior teeth (maxillary and mandibular) in the buccal area (Arapostathis et al. 2010) since it cannot be adapted to the posterior part of the mouth. Given that the device is used for injection of insulin into the skin, it has a nozzle pressure of 3000 psi (Annex 33).
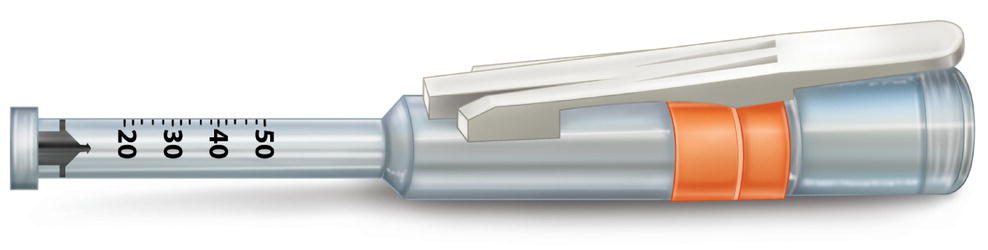
Figure 20.2 Injex®: high‐speed jet injection system.
Technique
- Preparation of the device for high‐pressure jet injection.
- Syrijet®. A 1.8‐ml dental cartridge is loaded. The dose is selected and the spring is activated to discharge.
- Injex®. The ampoule is loaded with the transporter and the spring is activated to inject with the “reset‐box.”
- Anesthetic solution. Any dental anesthetic solution can be used; the most common is the standard lidocaine with epinephrine. Epinephrine‐free solutions yield poorer results (Dabarakis et al. 2007); those with a higher concentration of anesthetic achieve better results, although they also lead to greater irritation of tissue (Lambrianidis et al. 1979–1980).
- Preparation of the patient.
- Show the device to the patient (Lambrianidis et al. 1979–1980; Saravia and Bush 1991; Munshi et al. 2000a).
- Inform the patient that during the discharge, he/she will hear a sound. We can avoid language with a high emotional content by not using the term “discharge,” but rather “jet.” Similarly, try to avoid the term “sound,” but rather euphemisms, such as “pop” or “click.”
- Advise the patient that he/she should not move during this stage.
- Dry and clean the area where the jet is to be applied (Bennett and Monheim 1971; Greenfield and Karpinsky 1973; Lambrianidis et al. 1979–1980; Saravia and Bush 1991).
- Apply the device to the mucosa.
- Hold the device so that it does not move during the discharge. Remember that the Syrijet® is fairly heavy (Bennett and Monheim 1971; Greenfield and Karpinsky 1973; Boj 1992).
- Place the mouth of the device against the attached mucosa and rest it on the bone to avoid tearing during the discharge (Greenfield and Karpinsky 1973; Boj 1992) and at a right angle, perpendicular to the point of discharge (Margetis et al. 1958; Kutscher and Zegarelli 1965; Bennett and Monheim 1971; Lambrianidis et al. 1979–1980) (Figure 20.3). Remember that it may be difficult to position the Syrijet® device correctly in the areas of the tongue and molars (Arapostathis et al. 2010).
- Discharge.
- Advise the patient to remain still and inform him/her that the process will only take a second (Stephens and Kramer 1964; Kutscher and Zegarelli 1965; Garellek 1967).
- The noise of the discharge causes the patient to react with a slight involuntary movement (Kutscher et al. 1964; Whitehead and Young 1968; Epstein 1971). He/she may also feel a slight jolt against the gingiva (Grau et al. 1997; Miegimolle et al. 2005).
- This maneuver is painless in 85% of cases (Table 20.1).
- The dose to be injected varies with the site:
- After the discharge:
- A small mucosal lesion with mild hematoma appears at the site of the discharge. There may sometimes be slight bleeding and mild blanching around the hematoma. Occasionally, we observe slight elevation of the area.
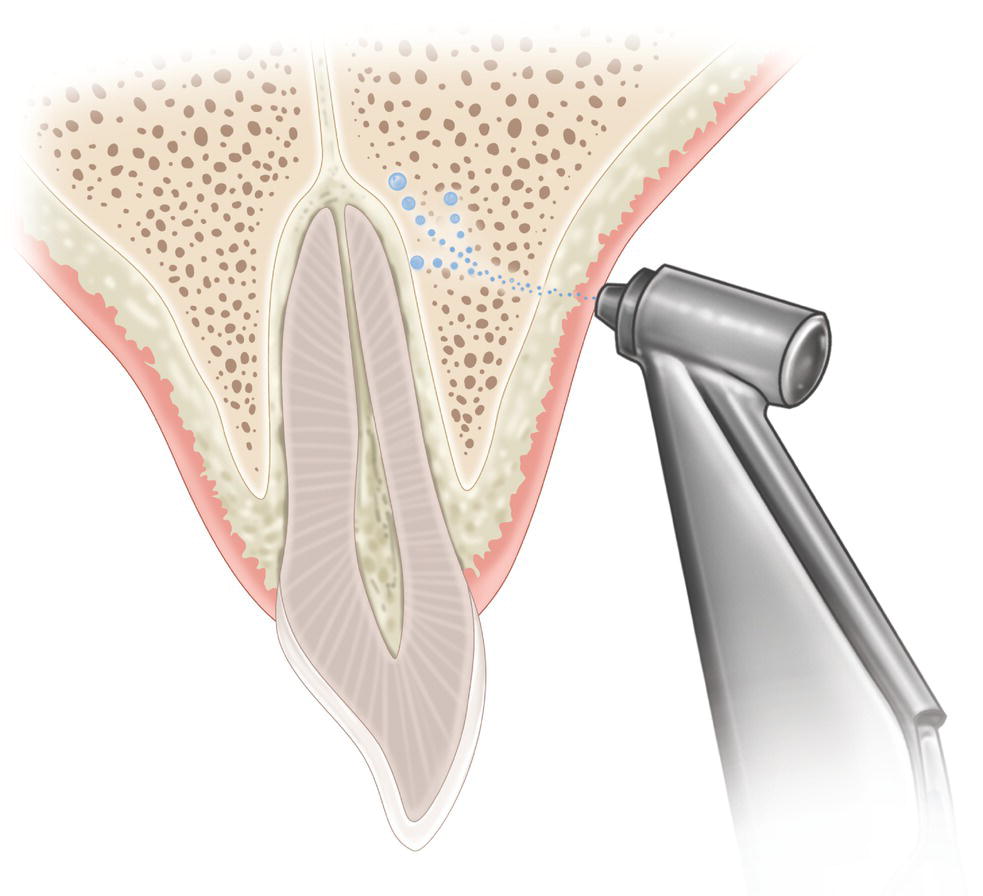
Figure 20.3 Discharge of Syrijet® with the nozzle of the jet supported against the inserted gingiva.
Source: Redrawn from Andlaw and Rock (1994).
- Wait 1–3 minutes (Kutscher et al. 1964; Stephens and Kramer 1964; Garellek 1967), sometimes 5 minutes (Lambrianidis et al. 1979–1980) to the onset of anesthesia.
- It is important to remember that the patient barely notices the soft tissue anesthesia (tingling, dullness, fat lip, etc.) (Stephens and Kramer 1964), although the area of anesthetized soft tissue can vary depending on the site:
- If anesthesia is insufficient, we have two options:
- Discharge more anesthetic, although more discharges and a larger quantity of anesthetic do not generally improve the results (Greenfield and Karpinsky 1973; Boj 1992).
- Inject more anesthetic using the conventional technique, with the advantage that the superficial‐topical anesthesia prevents the discomfort normally associated with the conventional injection.
- Preparation of the patient.
Complications of this Technique
- Bleeding and sometimes hematoma at the injection site in 35% of cases (Table 20.1), especially when several discharges are made at the same site (Greenfield and Karpinski 1972, 1973).
- Blanching in 90% of cases (Whitehead and Young 1968; Grau et al. 1997). This may be tender on palpation and inflamed (Margetis et al. 1958; Whitehead and Young 1968; Grau et al. 1997), especially when various discharges have been made at the same site (Greenfield and Karpinsky 1973).
- Laceration (cut in the mucosa) at the discharge site, especially when the patient moves his/her head as the discharge is made (Margetis et al. 1958; Kramer 1962; Stephens and Kramer 1964; Kutscher and Zegarelli 1965; Schmidt 1966; Garellek 1967).
- Bitter taste is reported if some of the anesthetic solution flows into the patient’s mouth. In some series, this occurs in more than 50% of cases (Arapostathis et al. 2010). To reduce the possibility of this complication, it is best to adapt the nozzle of the device to the tissues where the discharge is to be made.
- The patient may become startled owing to the surprise at the noise of the discharge, especially with Injex®. This occurs in around one‐third of cases (Dabarakis et al. 2007).
- Postoperative pain at the injection site in a small percentage of patients (Dabarakis et al. 2007). This usually resolves spontaneously in a few days.
Electronic Anesthesia: Electronic Dental Anesthesia
Electronic dental anesthesia (EDA) is a totally different system. We have previously discussed types of local anesthetic injection by various methods; however, EDA is based on electrical stimulation to generate anesthesia.
Transcutaneous electrical nerve stimulation (TENS) is used in general medicine. In dentistry, TENS is used to control chronic pain such as trigeminal neuralgia or atypical facial pain (Yap and Ong 1996; Cho et al. 1998). EDA is a variant of TENS that is used in dentistry to achieve anesthesia. It is based on a lower current and higher frequency (Cho et al. 1998).
The first references to the use of electrodes to relieve dental pain were by James Ferguson in 1770 (Ferguson 1770), although in 1858, the College of Dentists of London advised against the technique because electricity did not have an anesthetic effect and increased pain, and the few favorable outcomes achieved were the result of “distraction” (Kane and Taub 1975). However, operative systems began to be developed after the publication of the gate control theory in 1965 (see Chapter 11) (Melzack and Wall 1965), with the appearance of the first clinical trials in dentistry (Shane and Kessier 1967; Brooks et al. 1970; Laster and Pressman 1975). The first practical system marketed for dental use was UltraCalm, which appeared in 1989. The system was very expensive and required placement of an intraoral electrode in the area of the vestibule to be treated (Clark et al. 1987; Malamed et al. 1989). It was therefore necessary to dry the mucosa well to ensure successful placement (Cho et al. 1998). In addition, the electrode blocked the dentist’s vision and hampered the procedure (Cho et al. 1998; Baghdadi 1999). The other electrode was placed in the patient’s hand.
In 1993, the company 3M marketed a smaller device for use in dentistry known as the Dental Electronic Anesthesia System 8670 (Burke 1997; Baghdadi 1999). This was much more affordable and had the huge advantage that it involved placement of electrodes extraorally on the patient’s face. The system was introduced in the United Kingdom in 1997 (Burke 1997).
Mechanism of Action
The mechanism of action of EDA is unknown, although several factors have been shown to be associated with it (Munshi et al. 2000b):
- The gate control theory blocks the transmission of painful messages to the highest levels of the central nervous system (CNS) (Katch 1986; Clark et al. 1987; Hochman 1988; Silverstone 1989) (see Chapter 11, instrumental, vibrators).
- Release of serotonin, a neurotransmitter that is derived from tryptophan (Clark et al. 1987; Hochman 1988; Silverstone 1989; Cho et al. 1998). Adding 2–3 g of tryptophan to the diet, per day, 3 days before TENS is applied can approve the results (Hochman 1988).
- Release of β‐endorphins by the periaqueductal gray substance of the CNS (Clark et al. 1987; Hochman 1988; Silverstone 1989; Yap and Ong 1996; Cho et al. 1998). Endorphins are endogenous opioids and can produce analgesia.
- Placebo effect, because the patient controls the intensity of the current applied with his/her hand, thus keeping him/her distracted (Hochman 1988; Silverstone 1989; Mellor 1993; Modaresi et al. 1996).
Indications
- Conventional indications:
- Topical anesthesia, since this is very useful for reducing the pain resulting from the needle prick and injection of conventional anesthesia (Croll and Simonsen 1994; Quarnstrom and Libed 1994; Meechan and Winter 1996; Vongsavan and Vongsavan 1996; Meechan et al. 1998).
- Calculus removal in sensitive teeth and scaling and root planing (Bishop 1986; Clark et al. 1987; Hochman 1988; Pirkner et al. 1995; Yap and Ong 1996; Burke 1997).
- Placement of rubber dams, especially if the prongs of the clamp are placed subgingivally (te‐Duits et al. 1993; Baghdadi 1999).
- Cavities of small or moderate, barely invasive obturations (Malamed et al. 1989; Yap and Ho 1996; Cho et al. 1998). According to Table 20.2, the efficacy is 80%, although it is important to take into account that successful cases include those in which patients experienced with mild discomfort or pain but were able to complete treatment without the addition of conventional local anesthetic by injection. Some authors report that outcomes are better for primary teeth than for permanent teeth (Cho et al. 1998).
- Cementing of fixed prosthesis in vital teeth (Yap and Ong 1996; Burke 1997).
- Special indications for this technique:
- Allergy to local anesthetics or their components because this technique does not involve drugs (Jedrychowski and Duperon 1993; Yap and Ong 1996; Burke 1997; Munshi et al. 2000b).
- Hemophiliac patients because there is no need for an injection and, above all, there is no need for truncal block, with the result that there is no risk of asphyxiating hematomas (Savage 1982).
Note: It is noteworthy that EDA is more successful in children aged 5–12 years; in contrast, the results are not so good in anxious and/or skeptical patients (Quarnstrom and Quinn 1995).
Table 20.2 Percentage of patients in whom cavity cutting is successful (mildly aggressive) and who prefer EDA to conventional systems (results from clinical trials).
| Study data | Variables analyzed | |||
|---|---|---|---|---|
| Reference | Device | Patients | Successful cavities | Preference |
| Bradley et al. (1974) | Special air turbinea | Adults | 75% | — |
| Savage (1982) | HM‐100 PSU | Adults | 92% | — |
| Clark et al. (1987) | HFNM | Adults | 93% | — |
| Donaldson et al. (1989) | TENS | Adults | 33% | — |
| Malamed et al. (1989) | EDA | Adults | 86% | — |
| Esposito et al. (1993) | UltraCalm | Adults | 80% | 70% |
| Jedrychowski and Duperon (1993) | UltraCalm | Children | 83% | — |
| Mellor (1993) | UltraCalm | Adults | 100% | 60% |
| te‐Duits et al. (1993) | Spectrum Max‐SD | Children | 78% | 78% |
| Pirkner et al. (1995) | DEAS 3M (8670) | Adults | — | 56% |
| Sasa and Donly (1995) | DEAS 3M (8670) | Children | — | 40% |
| Segura et al. (1995) | DEAS 3M (8670) | Children | — | 93% |
| Jones and Blinkhorn (1996) | Cedeta | Children | 73% | 61% |
| Yap and Ho (1996) | DEAS 3M (8670) | Adults | 93% | — |
| Burke (1997) | DEAS 3M (8670) | Adults | — | 41% |
| Öztas et al. (1997) | U‐TENS plus | Children | 68% | 56% |
| Cho et al. (1998) | DEAS 3M (8670) | Children | 81% | 63% |
| Baghdadi (1999) | DEAS 3M (8670) | Children | 75% | 53% |
| Munshi et al. (2000b) | MES | Children | 94% | — |
| Average | 80% | 61% | ||
| Rounded average | 80% | 60% | ||
HFNM, high‐frequency neural modulator; MES, Madras Engineering Services; TENS, transcutaneous electrical nerve stimulation; EDA, electronic dental anesthesia; DEAS, Dental Electronic Anesthesia System.
a Miniature electrical generator within turbine head.
Disadvantages
This technique has several disadvantages, the most important of which are as follows:
- Inadequate depth of anesthesia (Harvey and Elliott 1995; Sasa and Donly 1995; Modaresi et al. 1996; Yap and Ong 1996; Burke 1997). Consequently, outcomes in oral surgery and extractions are poor (Bishop 1986; Katch 1986; Clark et al. 1987), as are those of endodontic procedures (Bishop 1986; Clark et al. 1987), cutting of crowns and bridges in vital teeth (Hochman 1988; Burke 1997), and deep scaling and root planing (Pirkner et al. 1995). Furthermore, the technique is not recommended in procedures where moderate or severe postoperative pain is expected (surgery, endodontics) (Malamed et al. 1989).
- The cost of the system and devices. While this has decreased over time, it continues to be high compared to traditional local anesthetic. In addition, single‐use adhesive electrodes are necessary for each new patient.
- The time taken to explain the technique to the patient and to try it, given that the patient’s cooperation is necessary (Modaresi et al. 1996; Yap and Ong 1996; Burke 1997).
Advantages
The advantages of the technique can be summarized as follows:
- No needle or injection, with the result that:
- There are no injections to fear (Jedrychowski and Duperon 1993; Yap and Ong 1996; Burke 1997). Some authors recommend this technique in patients with needle phobia (Hochman 1988; Malamed et al. 1989; Burke 1997; Munshi et al. 2000b), however, efficacy was not as well established in anxious or skeptical patients (Quarnstrom and Quinn 1995).
- It can be used in patients with bleeding dyscrasias because there are no injections. Moreover there is no need for truncal block and no risk of hematoma (Savage 1982), as seen previously.
- Patients prefer this technique to conventional techniques in 60% of cases, when it can be used (Table 20.2).
- Since no drugs are involved (local anesthetics, vasoconstrictors), there are no associated risks (Jedrychowski and Duperon 1993; Yap and Ong 1996; Burke 1997; Munshi et al. 2000b) such as the following:
- Toxicity induced by accidental intravascular injection or overdose.
- Allergy to any of the components in the anesthetic solution. This is one of the indications for this technique.
- No long‐term postoperative paresthesia (Hochman 1988; Malamed et al. 1989; Silverstone 1989; Jedrychowski and Duperon 1993; Yap and Ong 1996; Burke 1997; Munshi et al. 2000b) since the effect disappears once the device is switched off and there is no electric current:
- There is no risk of self‐injury resulting from biting the lips, tongue, or jugal mucosa or of burning oneself with hot food.
- The patient can eat and drink after treatment.
- Bite adjustment is easier because the patient’s perception is not altered by the anesthesia of the soft tissues.
Contraindications
While some contraindications are well established, others are empirical. However, in case of doubt, we include all possibilities:
- Abnormalities of the heart:
- Pacemaker, to prevent electromagnetic interference (Katch 1986; Hochman 1988; Donaldson et al. 1989; Malamed et al. 1989; Croll and Simonsen 1994; Quarnstrom and Libed 1994; Yap and Ho 1996; Yap and Ong 1996; Burke 1997; Meechan et al. 1998).
- Arrhythmias, to prevent alterations to heart rate caused by electrical depolarization (Donaldson et al. 1989).
- Abnormalities of the CNS and head:
- Cerebrovascular abnormalities such as stroke, transient ischemic attacks, and aneurysms since EDA can increase the risks of these occurring (Katch 1986; Hochman 1988; Donaldson et al. 1989; Malamed et al. 1989; Croll and Simonsen 1994; Quarnstrom and Libed 1994; Yap and Ho 1996; Yap and Ong 1996).
- Epileptic seizures or convulsions, owing to the risk of triggering them (Katch 1986; Hochman 1988; Quarnstrom and Libed 1994; Yap and Ho 1996; Yap and Ong 1996; Burke 1997; Meechan et al. 1998).
- Brain tumors, owing to the risk of worsening them (Croll and Simonsen 1994; Yap and Ho 1996).
- Neuralgia of the head and neck such as trigeminal neuralgia, postherpetic neuralgia, multiple sclerosis, Bell’s palsy, owing to the risk of worsening them (Croll and Simonsen 1994; Yap and Ho 1996; Yap and Ong 1996; Burke 1997; Meechan et al. 1998).
- Cochlear implants (Croll and Simonsen 1994; Yap and Ho 1996).
- Pregnancy, mainly because the effects on the fetus are unknown (Katch 1986; Hochman 1988; Donaldson et al. 1989; Malamed et al. 1989; Croll and Simonsen 1994; Quarnstrom and Libed 1994; Yap and Ho 1996; Yap and Ong 1996; Burke 1997; Meechan et al. 1998).
- Placement of electrodes is totally contraindicated at the following sites:
- Skin on the face affected by abnormalities (Croll and Simonsen 1994; Yap and Ho 1996; Yap and Ong 1996; Burke 1997).
- Eyes (Katch 1986; Burke 1997).
- Neck, because of stimulation of the carotid baroreceptors may induce bradycardia and subsequent reductions of cardiac output may not be tolerated in some patients (Yap and Ong 1996).
- Other possible contraindications:
- Fear of electrocution in patients who previously had a serious electrical accident (Quarnstrom and Libed 1994).
- Patients with communication barriers since they must understand the instructions to be able to cooperate (Donaldson et al. 1989; Yap and Ong 1996; Burke 1997).
- Arterial hypotension or bradycardia because EDA can worsen it (Hochman 1988).
Equipment
Annex 33 shows the main devices used in dentistry. Below, however, we refer to the Dental Electronic Anesthesia System 8670, manufactured by 3M Dental (Figure 20.4), since this was specially designed for dental anesthesia. Its technical features are shown in Table 20.3.
The device has a modern and attractive design, uses a 9 V battery, and is easy to handle, with manual controls:
- Switch, with three positions: M transmits smooth intermittent impulses, R transmits impulses in bursts, and C transmits continuous impulses and is the most commonly used option.
- Two buttons: R to set the frequency at 140 Hz and W to set the pulse width at 250 μs.
- Current intensity controller. Controlled by the patient, with a current that ranges from 0 to 60 milliamperes (mA).
- “On” button. When the device is turned on, a LED light advises us that the device is operational.
At both sides of the main box, we can find an input for the cables of the two electrodes. These electrodes are extraoral (this is an advantage because they do not interfere with interventions in the mouth) and are attached by means of spongy adhesive patches: green, main electrode, which is placed on the skin above the treatment area; brown, complementary electrode. The cables of the electrodes are reusable, therefore they should be cleaned after use. Each one goes with its respective color (green or brown).
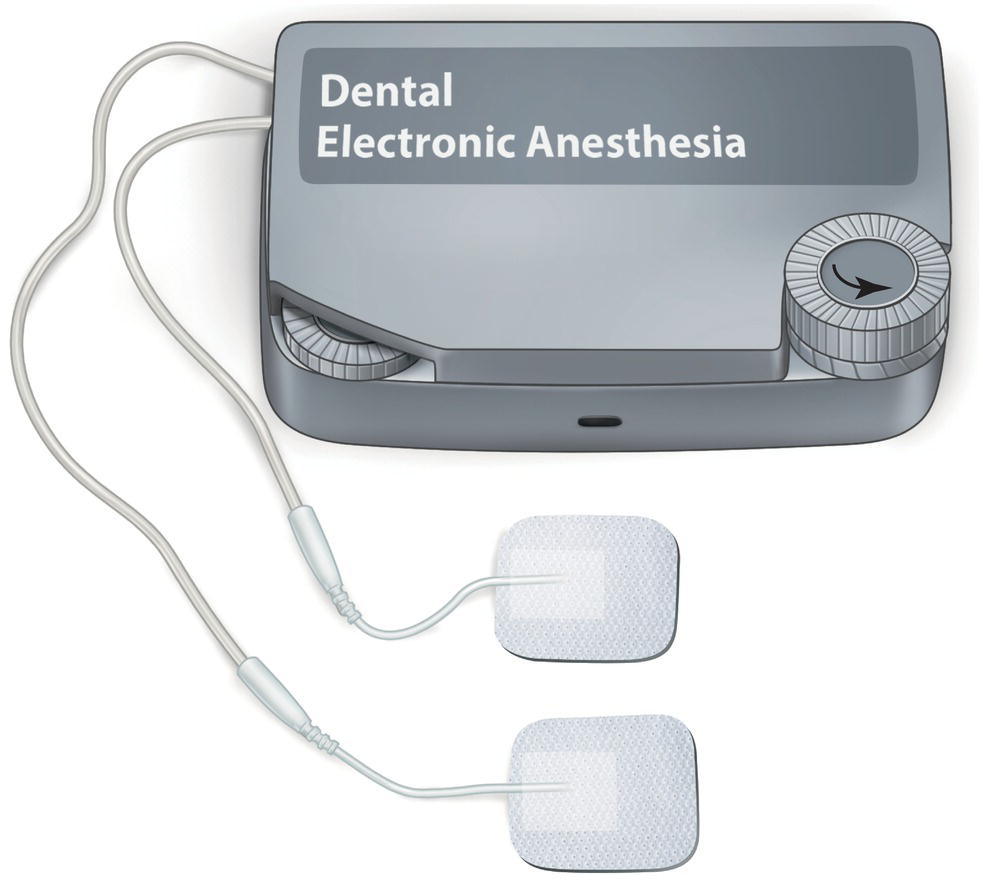
Figure 20.4 Dental Electronic Anesthesia System 8670 device from 3M dental, with adhesive extrabuccal electrodes.
Table 20.3 Technical characteristics of the Dental Electronic Anesthesia System 8670 from 3M Dental.
Source: Data obtained from: Croll and Simonsen (1994), Yap and Ong (1996), Burke (1997), Domínguez et al. (1998), and Cho et al. (1998).
| Variable | Measurement | Value |
|---|---|---|
| Cycle frequency | Hertz (Hz) | 140 |
| Current/amplitude | Milliamperes (mA) | 0–60 |
| Volts | Volts (V) | 9 |
| Bandwidth/pulse | Microseconds | 250 |
Technique
As stated above, here we address the Dental Electronic Anesthesia System 8670, manufactured by 3M Dental.
- Pre‐operative phase. Explain to the patient how the device works and show him/her the equipment and the single‐use adhesive electrodes.
- Children. While the child is in the dental chair, apply the “tell‐show‐do” technique and show pictures of children with the electrodes. Let the child touch the device and tell him/her that it “gives you tickles on your face” (Domínguez et al. 1998), which is a nice way of describing paresthesia.
- Place the extraoral adhesive electrodes (with the device off):
- Clean the skin of the face with alcohol to remove grease and sweat that might interfere with the transmission of the electrical current.
- Instruct the patient to open his/her mouth as wide as possible. This maneuver stretches the skin and brings the apex of the teeth closer. Thus, we can locate the area where the electrodes will be placed; the goal is to approximate the electrodes to the apices of the teeth being anesthetized.
- Place the adhesive electrodes on the skin. Each electrode symmetrically on each side of the skin, at least 1 mm apart.
- In the maxilla:
- Anterior teeth, more forward.
- Posterior teeth, more backward.
- Mandible, on the skin in the area of the chin:
- Anterior teeth, more forward.
- Posterior teeth, more backward.
- Try to ensure that the electrode is at the level of the apex of the tooth to be treated.
- Place the adhesive electrodes on the skin. Each electrode symmetrically on each side of the skin, at least 1 mm apart.
- Connect the electrodes to the cables on the box.
- Green connector. For the green electrode in the area of the apice of the teeth to be treated.
- Brown connector. For the electrode on the opposite side.
- Explain to the patient how to use the manual control (only the on–off button and intensity control button).
Note: In children aged under 8 years, the dentist and/or the assistant manages the device since children can increase intensity suddenly. While this does not damage tissue, it can be uncomfortable and surprise children, thus causing them to alter their behavior (Jedrychowski and Duperon 1993; Croll and Simonsen 1994).
- The patient should increase the intensity (mA) little by little until he/she feels a slight tingling sensation (Clark et al. 1987; Croll and Simonsen 1994). Small muscle contractions (fasciculations) also appear on the face and muscles near the electrodes (sign that the minimum therapeutic level has been reached).
- The patient can increase the intensity during the treatment. Most do so to levels 10–20 in 3–5 minutes. Smooth increases every 20 seconds are recommended.
- If the effect of the anesthesia is not sufficient during treatment, then we can use the following:
- Nitrous oxide (N2O/O2), which provides sedation and enhances analgesia (Donaldson et al. 1989; Croll and Simonsen 1994).
- Let the children listen to their favorite music through headphones, thus providing a distraction and increasing the pain threshold (Croll and Simonsen 1994).
- Administer injections of conventional local anesthetic, which do not hurt now because the patient is already feeling the anesthetic effect of EDA (Croll and Simonsen 1994; Quarnstrom and Libed 1994; Meechan and Winter 1996).
- When treatment has finished:
- Switch off the device. Warn the patient that he/she may feel fasciculations for another few minutes.
- Remove the electrodes from the skin of the face. The skin may be red. Explain to the patient that this will disappear in a few minutes (10–20 minutes).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


