I
Drug Class:
Bisphosphonate; calcium regulator
Dental Considerations
General:
• Bisphosphonates may increase the risk for osteonecrosis of the jaw (see section on “Medically Compromised Patients” for management considerations).
• Consider semisupine chair position for patient comfort if GI side effects occur.
• Patient may need assistance in getting into and out of dental chair. Adjust chair position for patient comfort.
Drug Class:
Mechanism of Action
Therapeutic Effect: Produces analgesic and antiinflammatory effects and decreases fever.
Indications and Dosages
Precautions and Contraindications
Possible increased risk for adverse cardiovascular events in patients at risk for thromboembolism
Drug Interactions of Concern to Dentistry
Serious Reactions
! Acute overdose may result in metabolic acidosis.
! Rare reactions with long-term use include peptic ulcer disease, GI bleeding, gastritis, a severe hepatic reaction (cholestasis, jaundice), nephrotoxicity (dysuria, hematuria, proteinuria, nephrotic syndrome), and a severe hypersensitivity reaction (particularly in patients with systemic lupus erythematosus or other collagen diseases).
Dental Considerations
General:
• Patients on chronic drug therapy may rarely have symptoms of blood dyscrasias, which can include infection, bleeding, and poor healing.
• Assess salivary flow as a factor in caries, periodontal disease, and candidiasis.
• Avoid prescribing aspirin-containing products.
• Consider semisupine chair position for patients with arthritic disease.
• Severe stomach bleeding may occur in patients who regularly use NSAIDs in recommended doses, when the patient is also taking another NSAID, anticoagulant/antiplatelet drug, or steroid drug, if the patient has GI or peptic ulcer disease, if they are 60 years or older, or when NSAIDs are taken longer than directed. Warn patients of the potential for severe stomach bleeding.
Consultations:
• In a patient with symptoms of blood dyscrasias, request a medical consultation for blood studies and postpone dental treatment until normal values are reestablished.
• Medical consultation may be required to assess disease control.
• Increased risk of adverse effects in patients with a history of thromboembolism, stroke, MI.
ibuprofen + famotidine
eye-byoo-proe′-fen & fa-moe′-ti-deen
Drug Class:
Nonsteroidal antiinflammatory drug (NSAID), histamine H2 antagonist
Precautions and Contraindications
Avoid use in patients with hypersensitivity to H2-receptor antagonists; history of asthma, urticaria, or allergic-type reaction to aspirin or other NSAIDs; perioperative pain in the setting of coronary artery bypass graft (CABG) surgery; late stages of pregnancy (>30 wk). Use with caution in patients with hepatic impairment, hypertension, and renal impairment. May increase the risk of hyperkalemia. May cause serious adverse skin events including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Use is contraindicated for treatment of perioperative pain in the setting of CABG surgery. Risk of MI and stroke may be increased with use following CABG surgery.
Drug Interactions of Concern to Dentistry
• NSAIDs, aspirin: increased toxicity of Duexis
• Reduced absorption of azole antifungal drugs (e.g., ketoconazole)
• Reduced antiplatelet effect of aspirin
• Aminoglycoside antibiotics: increased risk of renal failure
• Corticosteroids: increased risk of gastrointestinal ulceration and bleeding
• Reduced effectiveness of antihypertensive medications (e.g., thiazide diuretics)
Serious Reactions
! NSAIDs are associated with an increased risk of adverse cardiovascular thrombotic events, including fatal MI and stroke. NSAIDs may increase risk of gastrointestinal irritation, inflammation, ulceration, bleeding, and perforation. These events can be fatal and may occur at any time during therapy and without warning.
Dental Considerations
General:
• Duexis is not indicated for short-term management of acute dental pain.
• Consider use of non-NSAID pain relievers (e.g., acetaminophen, opioids).
• Patients taking Duexis are at increased risk of intraoperative and postoperative bleeding.
• Administration of other NSAIDs to patients taking Duexis can result in serious NSAID toxicity, including gastrointestinal ulceration and hemorrhage, thromboembolism.
• Monitor vital signs at every appointment due to adverse cardiovascular effects.
• Increased risk of adverse effects in patients with a history of thromboembolism, stroke, MI.
Drug Class:
Indications and Dosages
Serious Reactions
! Sustained polymorphic ventricular tachycardia, occasionally with QT prolongation (torsades de pointes) occurs rarely.
! Overdose results in CNS toxicity, including CNS depression, rapid and gasping breathing, and seizures.
! Expect prolongation of repolarization may be exaggerated.
! Existing arrhythmias may worsen or new arrhythmias may develop.
idarubicin hydrochloride
eye-dah-roo′-bi-sin high-droh-klor′-ide
Do not confuse idarubicin with doxorubicin, or Idamycin with Adriamycin.
Drug Class:
Anthracycline antibiotic; antineoplastic
Precautions and Contraindications
Preexisting arrhythmias, cardiomyopathy, myelosuppression, pregnancy, severe CHF
Serious Reactions
! Myelosuppression may cause hematologic toxicity (manifested principally as leukopenia and, to a lesser extent, anemia and thrombocytopenia), usually within 10–15 days of starting therapy.
! Blood counts typically return to normal levels by the third week.
! Cardiotoxicity (either acute, manifested as transient ECG abnormalities, or chronic, manifested as CHF) may occur.
Dental Considerations
General:
• If additional analgesia is required for dental pain, consider alternative analgesics (acetaminophen) in patients taking opioids for acute or chronic pain.
• Examine for oral manifestation of opportunistic infection.
• This drug may be used in the hospital or on an outpatient basis. Confirm the patient’s disease and treatment status.
• Chlorhexidine mouth rinse prior to and during chemotherapy may reduce severity of mucositis.
• Patient on chronic drug therapy may rarely present with symptoms of blood dyscrasias, which can include infection, bleeding, and poor healing. If dyscrasia is present, caution patient to prevent oral tissue trauma when using oral hygiene aids.
• Palliative medication may be required for management of oral side effects.
Consultations:
• Consult physician; prophylactic or therapeutic antiinfectives may be indicated if surgery or periodontal treatment is required.
• Medical consultation may be required to assess immunologic status during cancer chemotherapy and determine safety risk, if any, posed by the required dental treatment.
• Medical consultation may be required to assess disease control and patient’s ability to tolerate stress.
Teach Patient/Family to:
• Be aware of oral side effects.
• Encourage effective oral hygiene to prevent soft tissue inflammation.
• Report oral lesions, soreness, or bleeding to dentist.
• Prevent trauma when using oral hygiene aids.
• Update health and medication history if physician makes any changes in evaluation or drug regimens; include OTC, herbal, and nonherbal remedies in the update.
Drug Class:
Drug Class:
Alkylating agent; antineoplastic
Serious Reactions
! Hemorrhagic cystitis with hematuria and dysuria occurs frequently if a protective agent (mesna) is not used.
! Myelosuppression, characterized by leukopenia and, to a lesser extent, thrombocytopenia occurs frequently.
! Pulmonary toxicity, hepatotoxicity, nephrotoxicity, cardiotoxicity, and CNS toxicity (manifested as confusion, hallucinations, somnolence, and coma) may require discontinuation of therapy.
Dental Considerations
General:
• Determine why patient is taking the drug.
• If additional analgesia is required for dental pain, consider alternative analgesics (NSAIDs) in patients taking narcotics for acute or chronic pain.
• Examine for oral manifestation of opportunistic infection.
• Avoid products that affect platelet function, such as aspirin and NSAIDs.
• This drug may be used in the hospital or on an outpatient basis. Confirm the patient’s disease and treatment status.
• Chlorhexidine mouth rinse prior to and during chemotherapy may reduce severity of mucositis.
• Patient on chronic drug therapy may rarely present with symptoms of blood dyscrasias, which can include infection, bleeding, and poor healing. If dyscrasia is present, caution patient to prevent oral tissue trauma when using oral hygiene aids.
• Palliative medication may be required for management of oral side effects.
• Short appointments and a stress-reduction protocol may be required for anxious patients.
• Patients may be at risk of bleeding; check for oral signs.
• Oral infections should be eliminated and/or treated aggressively.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 Osteoporosis
Osteoporosis
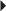 Acute or Chronic Rheumatoid Arthritis, Osteoarthritis, Migraine Pain, Gouty Arthritis
Acute or Chronic Rheumatoid Arthritis, Osteoarthritis, Migraine Pain, Gouty Arthritis Mild-to-Moderate Pain, Primary Dysmenorrhea
Mild-to-Moderate Pain, Primary Dysmenorrhea Fever, Minor Aches, or Pain
Fever, Minor Aches, or Pain Juvenile Arthritis
Juvenile Arthritis
 NSAID-Associated Ulcer Prophylaxis During Treatment for Osteoarthritis/Rheumatoid Arthritis
NSAID-Associated Ulcer Prophylaxis During Treatment for Osteoarthritis/Rheumatoid Arthritis Rapid Conversion of Atrial Fibrillation or Flutter of Recent Onset to Normal Sinus Rhythm
Rapid Conversion of Atrial Fibrillation or Flutter of Recent Onset to Normal Sinus Rhythm AML
AML Dosage in Hepatic or Renal Impairment
Dosage in Hepatic or Renal Impairment Hunter Syndrome
Hunter Syndrome Germ Cell Testicular Carcinoma
Germ Cell Testicular Carcinoma