Chapter 8
Systemic complications of endodontic infections
Introduction
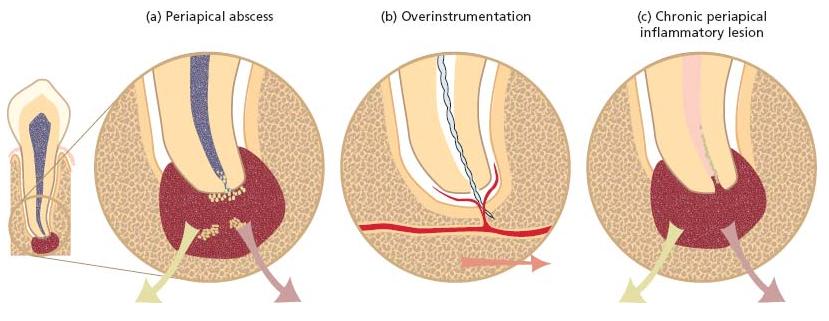
Infectious processes associated with the root canal system of teeth may give rise to various complications that not only result in local manifestations but may also produce lesions in other body sites. As outlined in Fig. 8.1, there are three means by which a root canal infection may cause metastatic infections:
The clinical significance of these mechanisms for the spread of infection and the measures to be undertaken to prevent systemic complications in otherwise healthy patients or patients compromised by a systemic disease are discussed in this chapter.
Acute periapical infections as the origin of metastatic infections
Acute manifestations of endodontic lesions involve the formation of abscesses in the periapical tissues (Chapter 7). Although these lesions are often confined to the oral region, they may extend to both nearby and distant body compartments along anatomical pathways (fascial planes and spaces). Hence, a periapical abscess may spread and reach the maxillary sinuses, the brain, the cavernous sinus, the eye or the mediastinum. Needless to say, some of these conditions are truly life threatening. In addition to the direct spread of pus and bacterial components, brain and lung abscesses may be caused by septic emboli. Furthermore, oral bacteria involved in endodontic infections may be aspirated into the lung and cause serious infections. Acute osteomyelitis is yet another condition that can arise from an endodon-tic infection. Before the antibiotic era, all these non-oral infections caused by disseminating oral bacteria were often fatal. In contrast to the status in developing countries, complications of this nature are now rare in the industrialized world. Yet, when they do occur, they still represent a threatening situation that demands proper dental and medical attention.
Fig. 8.1 (a) A periapical abscess at a root tip, (b) a root canal overinstrumentation and (c) an established periapical inflammatory lesion.
Spread of oral microorganisms by the circulation
Invasion of the circulation by bacteria and their dissemination by the blood stream throughout the body is called bacteremia. Bacteremias may occur as a result of surgical and other invasive procedures. They are generally asymptomatic and transient (duration <15–30 min) because the number of bacterial cells in the blood is usually low (<10 colony-forming units per ml). The host’s mononuclear phagocytes and the humoral immune response, furthermore, readily eliminate the organisms. Therefore, in healthy individuals transient bacteremias are usually of no clinical significance and are asymptomatic. However, in individuals who lack normal protection against infections (compromised hosts) the bacteria may start to multiply in the blood resulting in sepsis, a serious infection that is accompanied by systemic manifestations of inflammation. In compromised hosts (e.g. patients with cancer, unregulated diabetes or immunodeficiency), sepsis may proceed to a generalized fatal infection.
Oral microorganisms may gain access to the blood after loss of oral mucosal integrity from trauma or manipulation. In connection with endodontic treatment procedures, for example, the placement of a rubber dam clamp often causes transient bacteremia. Bacteremias may also follow instrumentation of root canals (see below). Bacteremia can occur spontaneously as well as in conjunction with various types of professional dental treatments and other oral manipulations, including oral health procedures and mastication (Table 8.1).
It is important to note that bacteremia occurs frequently from routine daily oral activities. In fact, bacteremias are 1000–8000 times more likely to be caused by daily oral manipulations than by dental treatment procedures (42). The incidence and magnitude of bacteremias of oral origin have been found to be directly proportional to the degree of oral inflammation and infection (9, 41) and occur more frequently in persons with high dental plaque scores and gingivitis than in individuals maintaining adequate oral hygiene (48). Calculations have been done to estimate how different types of dental treatment and oral manipulations may lead to risk of exposure to transient bacteremia. Roberts (47) estimated that tooth brushing twice daily for 1 year had a 154 000 times greater risk of exposure to bacteremia than that resulting from a single tooth extraction. He also compared a single tooth extraction and the cumulative exposure during 1 year to bacteremia from routine, daily activities and estimated the latter to be as high as 5.6 million times greater. One study in which the gingival health and plaque scores were the same in both test groups, found that the Sonicare electronic toothbrush induced significantly more (78%) bacteremias than did conventional toothbrushes (10).
Table 8.1 Frequency of treatment-induced and self-induced transient bacteremias.
| Dental procedure | Frequency of bacteremias | Reference |
| Intraligamental anesthetic injections in | 16–97 | 46 |
| children | ||
| Tooth extractions | 10–94 | 29 |
| Periodontal surgery | 36–88 | 19 |
| Gingival scaling | 8–80 | 19 |
| 25–61 | 2 | |
| Endodontics | 31–54 | 16 |
| 0–5 | 4 | |
| Ultrasonic scaling | 53 | 44 |
| Periodontal probing | 43 | 14 |
| Prophylaxis | 0–40 | 19 |
| Matrix band with wedge placement | 32 | 45 |
| Subgingival irrigation | 30 | 31 |
| Rubber dam clamp placement | 29 | 45 |
| Polishing teeth | 24 | 45 |
| Suture removal | 11–16 | 8 |
| Routine daily oral activities | ||
| Dental flossing | 0–58 | 19 |
| Chewing | 17–51 | 19 |
| Water irrigation device | 7–50 | 19 |
| Oral rinsing | 50 | 20 |
| Toothpicks | 20–40 | 9 |
| Tooth brushing | 0–26 | 19 |
Bacteremia and endodontic treatment
The actual number of microorganisms introduced into the blood stream depends upon the size of the apical foramen, the degree of infection of the root canal and the method of root canal treatment (2). A variety of oral bacteria, and species that are found in infective endocarditis, have been isolated from infected root canals and peri-apical lesions (27), yet there are relatively few reports in the literature describing how often bacteremia occurs following endodontic therapy and few provide bacteriological findings (Table 8.2).
Studies performed during the 1960s were not able to demonstrate positive blood cultures even if the root canal system had been instrumented vigorously in the presence of saliva. However, when canals were instrumented beyond the root apex, there was a 25–30% incidence of bacteremia (8). Baumgartner et al. (4) used an aseptic technique to culture the blood of 20 patients and registered bacteremia in only one case when a root canal had been overinstrumented. Debelian et al. (15), on the other hand, found a comparatively high frequency of bacteremias subsequent to endodontic therapy (42%), particularly in cases where the endodontic instrumentation had been deliberately carried out beyond the apical foramen (7/13 versus 4/13 for non-overinstrumented cases). In this latter study anaerobes were frequently isolated from the positive blood cultures, as opposed to previous studies where facultative organisms had predominated. These authors later verified that, for each patient in which a positive blood culture had been found, there was phenotypic and genotypic homology between the bacteria isolated from the root canal and the blood, suggesting that the blood bacteria originated from the treated root canals (16). Interestingly, in one patient the fungus Saccharomyces cerevisiae was recovered from both the root canal and the blood sample (Advanced concept 8.1).
Table 8.2 Studies showing bacteria isolated from blood samples obtained in conjunction with non-surgical or surgical endodontic therapy.
Infective endocarditis
Bacteremia is considered a risk factor for the development of endocarditis. Bacterial endocarditis is a bacterial infection of the heart valves and the epithelial lining (endocardium) of the heart. The term infective endocarditis has recently been proposed to emphasize the fact that microbes other than bacteria also may cause endocarditis (25). According to new terminology, infective endocarditis is named after the infective microorganism, e.g. streptococcal endocarditis, staphylococcal endocarditis or fungal endocarditis (Table 8.3). Although currently termed infective endocarditis, bacterial endocarditis is still used by many authors in the dental and medical literature.
Infective endocarditis results from a complex interaction among the endocardium, local hydrodynamic effects, circulating microorganisms and local and systemic host defense factors. In many countries it is a relatively uncommon life-threatening disease (approximately 50 cases are officially registered in Norway and 300 in Denmark per year). Infective endocarditis usually occurs in individuals with underlying congenital or acquired structural cardiac defects who develop bacteremia with bacteria prone to causing endocarditis. Symptoms of endocarditis generally start within 2 weeks of the incited bacteremia, although the time to diagnosis may be shorter or longer (51). An incubation period longer than 2 weeks between the invasive procedure and the onset of symptoms significantly lessens the likelihood of the procedure being the proximate cause (30).
The symptoms are non-specific and include fever, malaise, anorexia, cardiac murmurs, splenomegaly, anemia and weight loss. Before the antibiotic era the mortality of bacterial endocarditis was 100%, and it still is if not treated adequately. Currently, the death rate is less than 10% for viridans (alpha-hemolytic) streptococcal endocarditis (21, 55) and 30% for staphylococcal endocarditis (21).
The organism(s) in the circulation causing the disease adheres to and forms vegetations in a focal area of the heart valves. A prerequisite is often a prior injury where fibrin and platelets have been released, which can capture circulating microbes. Multiplication within the vegetations leads to discharges of the infecting organism(s) back to the circulation, producing a constant bacteremia that gives multiple positive blood cultures. The clinical symptoms, including embolization to organs, are a direct result of this mechanism.
Table 8.3 Relative frequency of oral viridans streptococci associated with infective endocarditis at the New York Hospital from 1944 to 1983 (51).
| Species | Frequency (%) |
| Streptococcus mitis | 33–41 |
| Streptococcus sanguis | 31–47 |
| Streptococcus anginosus | 5–8 |
| Streptococcus mutans | 3–10 |
| Streptococcus salivarius | 1–2 |
| Nutritionally variant | 6–7 |
| Unspeciated | 1–3 |
A wide variety of bacteria has been isolated from blood of patients with infective endocarditis. Viridans streptococci are the most common (50–63%) followed by staphy-lococci (25–26%) (21, 54). Various other microorganisms account for less than 10% (34). Among the oral viridans streptococci associated with infective endocarditis, Streptococcus mitis and Streptococcus sanguinis dominate and account for more than two-thirds of the registered cases.
The reason why viridans streptococci are more likely than other types of streptococci to cause endocarditis relates to their release of extracellular polysaccharides, which provides them with an exceptional adhesion mechanism. Other adhesins like lipoteichoic acid, fibrin-ogen-binding protein, fibronectin-binding protein and platelet-interactive molecules are putative virulence factors of bacteria associated with endocarditis (21). It was suggested recently that the majority of infected root canals contain bacteria that may have the potential to cause bacterial infective endocarditis (3).
Staphylococcus aureus is another important pathogen that may originate from the oral cavity, although there is no convincing evidence that oral staphylococci can cause infective endocarditis (59). However, this organism is capable of infecting even structurally normal heart valves and is the most commonly isolated organism in infective endocarditis of intravenous drug abusers (54).
It should be recognized that oral microorganisms presumed to cause infective endocarditis in a given case are not normally specific to the oral cavity only. Furthermore, the incubation period (the time between a procedure resulting in bacteremia and the onset of symptoms) is often well outside the accepted time frame, which should be within 10–14 days, depending on the causative organism (30). This means that it is often hard to establish the origin of a given heart infection.
Cardiac conditions and dental treatment procedures as risk factors for infective endocarditis
The American Heart Association (AHA) has made recommendations for the prevention of infective endocarditis for more than half a century. Nine documents were published from 1955 to 1997 (13, 58). During these years the guidelines became more and more complicated and increasingly difficult for both practitioners and patients to follow. Risk assessment of patients for infective endocarditis was based on types of heart diseases and oral intervention. The AHA now recommends that only patients at greatest risk of negative outcomes from infective endocarditis should take short-term preventive antibiotics before routine dental procedures. According to the AHA, patients who should continue to take preventive antibiotics include those with artificial heart valves, a history of infective endocarditis, certain congenital heart conditions (including a patch to repair the heart defect within the past 6 months) and a cardiac transplant that develops heart valve problems. Thus an extremely small number of cases of infected endocarditis might be prevented by antibiotic prophylaxis even if it were 100% effective (58).
Certain cardiac conditions are thought to predispose individuals for infective endocarditis more often than others. Most at risk are those patients with a prior history of infective endocarditis and those with a prosthetic heart valve. In line with this knowledge, the AHA (58) has defined high-risk and moderate-risk categories for infective endocarditis (Core concept 8.1). This body has also defined dental and oral treatment procedures that are likely to cause hazardous bacteremia in these two infective endocarditis categories (Core concept 8.2). Hence, a variety of invasive dental procedures is felt to pose a risk for infective endocarditis, although the associations have never been firmly documented. Endodontic surgery, including incision and drainage of abscesses and instrumentation beyond the tooth apex, belong to the dental procedures that, according to the AHA, should be regarded as risk factors to individuals with cardiac conditions.
- Prosthetic cardiac valve or prosthetic material used for cardiac valve repair
- Previous infective endocarditis
- Congenital heart disease (CHD):*
- Cardiac transplantation recipients who develop cardiac valvulopathy
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses