Chapter 7
Apical periodontitis
Introduction
Apical periodontitis is an inflammatory lesion in the periodontal tissues that is caused mostly by bacterial elements derived from the infected root canal system of teeth (Core concept 7.1). In non-treated teeth apical peri-odontitis represents a defensive response to a primary infection in a necrotic pulp. Apical periodontitis may also develop due to a secondary infection subsequent to endodontic treatment procedures. Post-treatment apical periodontitis is most commonly due to either unsuccessful control of primary root canal infection by endodontic treatment measures, or infection or reinfection of the root canal system due to inadequate obturation and/or inadequate coronal seal that allowed bacterial leakage to take place. Inadvertent extrusion of certain medicaments and root filling materials into the periapical tissue compartment may also cause tissue toxic effects as well as precipitate foreign body reactions (Chapter 12). In this chapter the focus will be on apical periodontitis associated with non-endodontically treated teeth affected by root canal infection. It will be described in terms of biological function, pathogenesis and clinical as well as histological presentation.
Fig. 7.1 Potential sites for emergence of endodontic lesions in the periodontium.
The nature of apical periodontitis
Apical periodontitis serves an important protective function, aimed at confining bacteria discharged from the root canal space and preventing them from spreading into adjacent bone marrow spaces and other remote sites. The process is unique in the sense that it cannot eradicate the source of infection. The reason is that once a pulp has become necrotic, defense mechanisms cannot operate far into the root canal owing to the lack of vascular support. Although these mechanisms can act at the apical margins of the necrotic tissue, they are unable to penetrate it in a fully developed tooth. Consequently, without proper endodontic treatment apical periodonti-tis may prevail chronically.
Bone resorption is a most conspicuous feature of apical periodontitis and is an unavoidable side-effect of the defensive process. Some may view it as a “price” paid by the host to provide the necessary, effective, immune response to the root canal infection. Bone loss that appears in radiographs serves as the main clinical indicator for the presence of apical periodontitis (Fig. 7.2c), as many of these lesions are silent and prevail without overt clinical symptoms. Such lesions will be referred to in this text as asymptomatic apical periodontitis. Asymptomatic apical periodontitis is mostly biofilm derived (39). Yet, acute forms do occur and may develop during the expanding phase of the initial lesion. Lesions of symptomatic apical periodontitis may also emerge as a result of a disturbed equilibrium between the host defense and the bacterial infection in an already established lesion. Symptomatic apical periodontitis results mostly from the action of planktonic bacteria (11, 39). Hence, single lesions of apical periodontitis can be symptomatic or asymptomatic at different stages of their development and progress ion. By far, most cases of apical periodontitis are asymptomatic.
Pain, tenderness to biting pressure, percussion or palpation as well as swellings are typical clinical expressions of symptomatic apical periodontitis (Fig. 7.2a, b). The symptoms may vary from mild to severe. More dramatic clinical symptoms of apical periodontitis may appear and dominate when the local immune defense has failed to detain the infection resulting in an apical abscess. Although very rare, acute apical periodontitis may develop into a very serious and even life- threatening condition. Phlegmonous spread of the infection into the connective tissue around the upper respiratory tract, orbit, neck or even to the brain is alarming. Infectious elements released in conjunction with symptomatic apical periodontits may also be distributed via the blood stream and cause heart valve and myocardial infections (see Chapter 8). Even though apical periodontitis may be associated with such severe clinical manifestations, it needs to be stressed that its basic function is to contain root canal bacteria and not allow their dissimenation to distant sites (Core concept 7.2). Identification of apical periodontitis is directly related to the resolution of our examination tools; many apical periodontitis lesions that are not seen on conventional radiographs are identified on CBCT (cone beam computed tomography) and many more may be identified only upon histological examination.
On a microscopic level, different structural frameworks of apical periodontitis can be identified. These forms include apical granuloma, apical abscess and apical cyst. These lesions will be described here in terms of their general histological features while their clinical presentation will be detailed later in the chapter. Clinically and radiographically these histopathological entities cannot be distinguished from each other or recognized, with the exception of abscesses with a sinus tract.
Apical granuloma is the most common form of apical periodontitis and consists of an inflammatory lesion dominated by lymphocytes, macrophages and plasma cells (see also Key literature 7.1 and Advanced concept 7.1) (Fig. 7.3). Numerous fibroblasts and connective tissue fibers are usually present with abundant capillaries. At its periphery an encapsulation attempt may often be found but great structural heterogeneity is the norm for apical periodontitis (31, 34).
Epithelial cell proliferation is a common finding in longstanding apical granulomas and may occur in up to 50% of the lesions (31, 33, 34, 45). The epithelium is believed to originate from the epithelial cell rests of Mallasez. Under the influence of cytokines and growth factors released in the inflammatory process, the normally resting cells divide and migrate. They may form more or less continuous strands that seem to take a random course (Fig. 7.4). They may also become attached to the root surface (Fig. 7.5).
Fig. 7.2 Different clinical presentations of apical periodontitis due to an infected necrotic pulp: (a) extraoral swelling in the left cheek region; (b) intraoral vesi-tibular swelling associated with the severely broken down first premolar; (c) apically positioned radiolucent area (upper left incisor).
Fig. 7.3 Cells of the periapical granulomas: (a) lymphocytes (Ly), macrophages (M) and connective tissue fibroblasts (F) are the three main cells in the periapical granuloma. (b) Other cells like PMNs are also found but they are newcomers, not residents of the granuloma: each of them was recently recruited and will die in a few days.
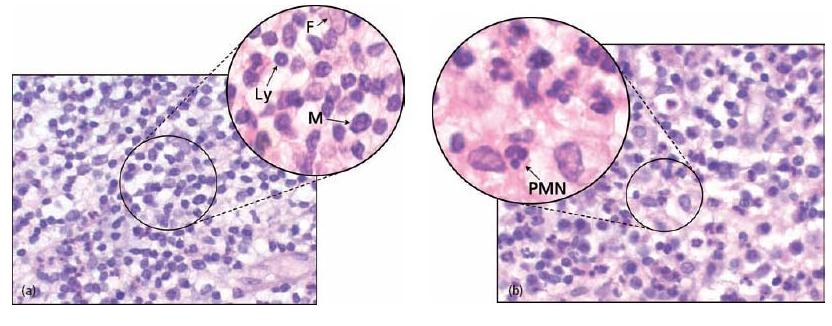
Fig. 7.4 Epithelial strands in a periapical granuloma. Epithelial cell masses, originating from the epithelial rests of Mallasez, proliferate in periapical granulomas and form strands. Some are cut longitudinally (A) and some perpendicular to the long axis of the strand (B).
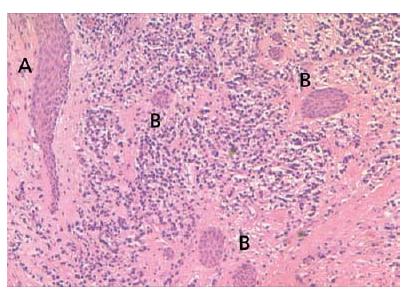
Fig. 7.5 Epithelial strands in a periapical granuloma that seem to attach to the root tip (arrows).
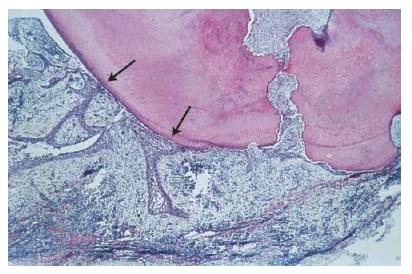
Apical abscess denotes the presence of pus within the lesion. Abscess formation may reflect a shift in cellular dynamics within a pre-existing apical granuloma or be a direct outcome of an acute primary infection. The influx of PMNs is now dramatically increased. Upon the intense phagocytic activity of these cells and upon their death, tissue-destructive elements (e.g. hydrolytic enzymes and reactive oxygen species) are released to the extent that macrophages are no longer able to keep up with clearing and repairing the cell and tissue damage induced. Connective tissue constituents such as collagen and hyaluronic acid are degraded and the tissue in the center of the lesion is liquefied. In the periphery, granuloma-tous tissue may persist.
Consequently, a continuum exists between apical abscesses and apical granulomas. While in some cases apical granulomas may contain only small numbers of infiltrating PMNs, other cases present with a massive influx of PMNs, leading to tissue liquefaction and pus formation.
An apical cyst is an epithelium-lined cavity that contains fluid or semi-solid material and is commonly surrounded by dense connective tissue variably infiltrated by mononuclear leukocytes and PMNs (Figs 7.6 and 7.7). The cyst cavity is most commonly lined with stratified squamous epithelium of varying thickness that originates from the epithelial rest cells of Malassez. Rarely, the lining may be ciliary epithelium originating from the adjacent maxillary sinus. The epithelial lining may be continuous, but may also be disrupted or even completely missing in certain areas of the cavity. It should be noted that apical cysts may also become abscessed.
In contrast to periapical granulomas, some apical cysts appear never to arrive at a steady state. They may slowly expand over time and eventually, if left untreated, may consume a considerable portion of the surrounding bone (Fig. 7.8).
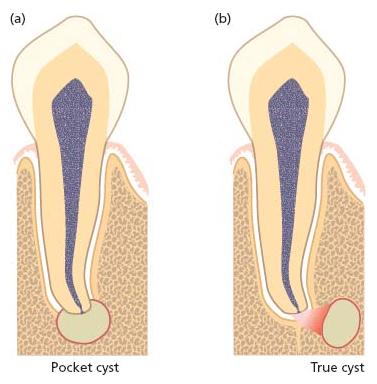
Apical cysts are divided into pocket cysts (bay cyst) and true cysts (29, 33, 34) (Fig. 7.9). A pocket cyst is an apical inflammatory cyst that contains a sac-like, epithelium-lined cavity that is open to and continuous with the root canal space. True apical cysts, on the other hand, are located within the periapical granuloma with no apparent connection between their cavity and that of the root canal space.
The mechanism by which cysts are formed, grow and expand is not well understood, but is most likely to involve inflammatory mediators that are present in the tissue lesion (13) (for cyst formation theories see also Advanced concept 7.2).
Fig. 7.6 Lining of a periapical cyst. Stratified squamous epithelium forms the lining of a periapical cyst which formed within a granuloma. A = granuloma; B = epithelial lining; C = cyst cavity.
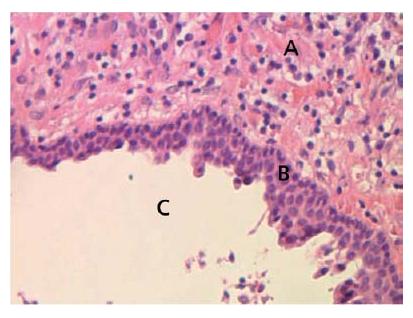
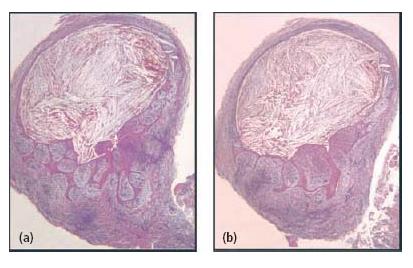
Fig. 7.7 Sections through a soft-tissue lesion encompassing an apical cyst. In this case, the cyst lumen was filled with cholesterol crystals. (From Ricucci and Bergenholtz (38). Reproduced with permission from Wiley-Blackwell.)
Given the presumed mechanisms of pathogenesis (Advanced concept 7.2), epithelial growth in some apical cysts ceases when the stimulating factors are eliminated, for example after proper endodontic treatment. Subsequently the epithelium lining may become thin and even disappear and thus provide conditions for healing.
In some cases both the cyst capsule and the cyst cavity may contain cholesterol, which forms oblong needle-like crystals (Fig. 7.7). In tissue sections the crystals are not seen but appear as typical tissue clefts resulting from the dissolution of the cholesterol during histological tissue processing. The crystals are formed in the connective tissue of the cyst capsule and are gradually moved towards and into the cyst cavity. They attract multinu-clear giant cells of the foreign body type and, thus, elicit a foreign body response in the connective tissue (28). The crystals are thought to derive from disintegrating red blood cells in stagnant vessels of the lesion. Inflammatory cells and circulating plasma lipids are other proposed sources (2).
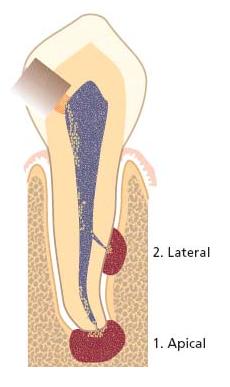
Fig. 7.8 Different clinical presentations of apical cysts. Radiographs in (a) and (b) demonstrate two radiolucent areas; one small associated with tooth 33 and one large with extension in a distal–coronal direction on tooth 34. Although the size and shape of a radiolucent lesion are not definitive criteria for cyst formation, there are other features suggestive of apical cyst. On opening tooth 33 for endodontic treatment, clear exudates drew off from the root canal (c). It was not possible to stop the exudation and this prevented completion of endodonic therapy. At the buccal and distal aspects of tooth 34 there was a distinct prominence that was hard and not tender to palpation (d). On raising a flap for enucleation of both processes, the expansive process is more clearly visible (e). Thin bone tissue limited the fluid-filled process at the surface. Histological examination of a tissue specimen confirmed the diagnosis.
Fig. 7.9 Radicular cysts may appear in two configurations: a pocket cyst (a) where there is direct communication between the cyst cavity and the root canal space; and a true cyst (b) where no such pathway exists.
Interactions with the infecting microbiota
The course and the severity of the tissue response to root canal infection depend on the microbial load (number of organisms and their pathogenic potential, see Chapter 6), the state of the defense potential of the host and time. Acute and severe forms may develop at initial stages when microorganisms have rapidly increased in numbers and overwhelmed the local immune defense. This scenario is related mostly to bacteria in their planktonic state, which allows fast growth of organisms. Certain pathogens seem prone to cause acute processes. Organisms belonging to the anaerobic segment of the microbiota, including genera of Porphyromonas, Prevotella, Fusobacterium and Peptostreptococcus, are more often associated with symptomatic and painful lesions of apical periodontitis than are other types of organisms (12, 46, 49).
Bacterial elimination
Bacterial elimination in periapical lesions is carried out by PMNs. All other constituents and processes in apical periodontitis may be viewed as serving this ultimate goal (Fig. 7.10). Local recruitment of PMNs from the capillaries, by ICAM-1-mediated margination, depends on inter-leukin-1 (IL-1) and tumor necrosis factor alpha (TNFα) derived from activated macrophages. Specific immuno-globulins are essential for effective phagocytosis. Their local production requires antigen presentation followed by activation of specific T-lymphocytes to produce a set of cytokines that will allow proliferation of antigen-specific B-lymphocytes and their maturation into specific IgG-producing plasma cells, and interferon gamma (INFγ) activates macrophages to produce the IL-1 and TNFα required for the above tasks, as well as IL-8 which furt/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses