Chapter 6
The microbiology of the necrotic pulp
Introduction
Breakdown of the dental pulp by any cause (Chapter 2) results in loss of defense mechanisms that can counter microorganisms in the oral cavity from entering the root canal system of teeth. In direct exposure by, for example, caries or fracture, microorganisms readily occupy the available pulpal space. In apparently intact teeth microorganisms may also find ways of accessing root canals, where the vital functions of the pulp have been lost (Fig. 6.1): the attractant is the necrotic tissue, which will serve as a primary nutrient for microbial growth and multiplication. The root canal space is also a sanctuary where colonizing microorganisms can build up microbial communities in the form of biofilms without much interference from the defense system of the host. Infection of root canals, described in detail in this chapter in terms of microbial biofilms, ecological determinants and pathogenic potential, will initiate and maintain inflammatory lesions in the periodontium. While this may occur at any portal of exit from the root canal system, these lesions are often established near the root tip and therefore go under the term apical periodontitis (see Chapter 7).
Evidence for the essential role of microorganisms in apical periodontitis
Historical background
Microorganisms colonizing the necrotic pulp of teeth have long been recognized as the cause of acute and chronic manifestations of apical periodontitis (Chapter 7). The first observation of microorganisms in root canals was by Antony van Leeuwenhoek (Key literature 6.1), whose home-made microscope also enabled him to make the first drawing of dental plaque in 1683. However, it was 200 years before root canal microorganisms came under biological investigation. Willoughby D. Miller (1853–1907), the father of oral microbiology, described the clinical effects of ‘gangrenous tooth pulps’ as centers of infections varying from hardly perceptible apical inflammation to severe local and general symptoms, sometimes even with fatal outcome (Key literature 6.1). He cultured and characterized bacteria from necrotic pulps and studied their pathogenic potential in animal experiments (40).
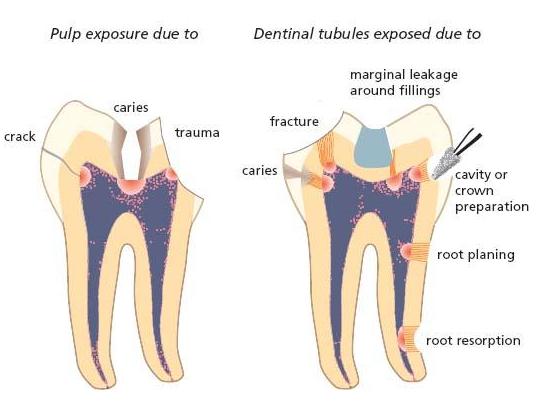
Fig. 6.1 Drawing illustrating the pathways of entry for microorganisms into the root canal. Obvious ways of entry are pulp exposures due to caries or trauma. Potential pathways are cracks in enamel and dentin due to trauma, and dentinal tubules exposed by caries, fracture, cavity or crown preparation, marginal leakage around filling, root resorption or root planing.
With the publication in 1911 of William Hunter’s book Oral Sepsis as a Cause of Disease (cited in Ref. 9), the theory of focal infection emerged: the concept that infected teeth cause infections in other parts of the body and also cause many systemic diseases. Hunter accused dentists of producing masses of oral sepsis with their procedures for fillings, crowns and bridges, which often caused pulpitis, pulp necrosis and apical periodontitis. He proposed that a more suitable name for ‘conservative dentistry’ was ‘septic dentistry’. His publication (26) led to the view that all teeth suspected of infection should be extracted. This concept gained widespread acceptance for years and resulted in mass extractions of teeth. It probably caused a delay in the development of modern endodontics, but eventually led to biologically sound treatment methods, including the elimination of root canal infections.
Observations in animal experiments and human studies
The view that microorganims in root canals play a key role in the pathogenesis of apical periodontitis has not always been universally shared. A primary reason has been failure to produce cultures from root canals in humans to confirm a clear-cut association of apical peri-odontitis with presence of bacteria in sampled teeth. Some early authors therefore suggested that decomposition of necrotic pulp tissue or stagnant tissue fluid in the pulpal space might cause apical periodontitis alone, in the absence of root canal infection. However, animal experiments proved this theory wrong and demonstrated that empty tubes or sterile dead tissue implanted subcu-taneously caused only transient inflammation that did not prevent healing, whereas necrotic tissue infected with bacteria caused intense inflammation and often abscess formation (35).
A key piece of evidence for the crucial role of microorganisms in the pathogenesis of apical inflammatory lesions was provided by the classical study by Kakehashi, Stanley and Fitzgerald in 1965 (28). These investigators used germ-free and conventional rats to demonstrate that only in the presence of bacteria could pulp necrosis and apical periodontitis be induced in teeth where pulps were exposed to the oral environment. In germ-free animals, on the other hand, the exposed pulps healed with hard-tissue repair at the exposure site in spite of gross food impaction. In agreement with this study, aseptically devitalized pulps that were sealed and kept sterile for 6–7 months in experimental monkeys did not result in inflammatory responses of the apical tissues. In contrast, pulps lacerated by instrumentation and contaminated with oral bacteria caused clinical, radiographic and histological signs of apical periodontitis (42).
Cultural studies in humans, when taking account of the fastidious and often obligate anaerobic nature of the root canal microbiota, were also eventually able to show a connection of apical periodontitis with the presence of bacteria in root canals. In these studies (6, 43, 63) nonvital tooth pulps with closed necrosis after trauma were accessed and sampled for bacterial culture under strict aseptic conditions. Indeed a strong association was confirmed in that absence of bacterial growth correlated with cases without radiographic signs of apical inflammation, while most cases with an obvious lesion gave bacterial growth.
Routes of microbial entry to the pulpal space
The root canals of teeth are normally sterile and the presence of microorganisms is dependent on their invasion. As long as the pulp is vital and has a functioning infection defense, bacterial entry will usually be opposed, especially in cases where there is no direct exposure of the pulpal tissue to the oral cavity (see Chapter 2). On the other hand, when a deep carious lesion reaches the pulp the massive bacterial exposure will eventually cause the inflammatory defense barrier to recede, thereby giving access for the bacteria at the caries front to invade and colonize the pulpal space. A similar route of entry will occur in teeth with pulp exposure due to trauma, fracture and cracks as well as following unprotected iat-rogenic exposure in restorative procedures. Direct pathways to the root canal space may be present in teeth with periodontal disease. From subgingival plaque, bacteria can enter through accessory lateral and furcal canals and, in ultimate periodontal breakdown, through the apical foramen.
A direct pathway from the external environment is not necessarily a prerequisite for microbial colonization of the pulpal space, however. In cases of a damaged pulp, oral microorganisms may enter along the dentinal tubules following their exposure by trauma, root resorption, root caries, gaps in the cementum in the cervical area and by cavity and crown preparation or under restorations with marginal gaps. Even in seemingly intact teeth following breakdown of the pulp, as in trauma cases (Chapter 15), microorganisms may cross the hard-tissue barrier (6, 63). In fact, a route of entry is possible through minor cracks in enamel and dentin that occurred in conjunction with the injury (34). Anachoresis has also been suspected of leading to infection in these cases. This term means that bacteria have been disseminated to the site of inflammation out of the blood stream. It is well known that bacteremia is common in humans (Chapter 8). Yet, bacteria normally are eliminated from the blood stream and the fact that species from other body sites are rarely recovered in traumatized teeth has cast the significance of anachoresis as a route of entry to necrotic pulps in doubt. Figure 6.1 summarizes the most common pathways for microorganisms in the oral cavity to enter the root canal in cases of pulp necrosis.
Modes of colonization
Microorganisms colonizing a body site such as the root canal space may either be free-floating as single cells (planktonic form) or attached to each other or on to the root canal walls or both. Organisms dwelling in a planktonic state require a liquid phase. In root canals the fluid is primarily inflammatory exudate that is released at the host defense–bacterial front interface. Saliva may also be the vehicle where there is direct communication of the root canal system with the oral environment.
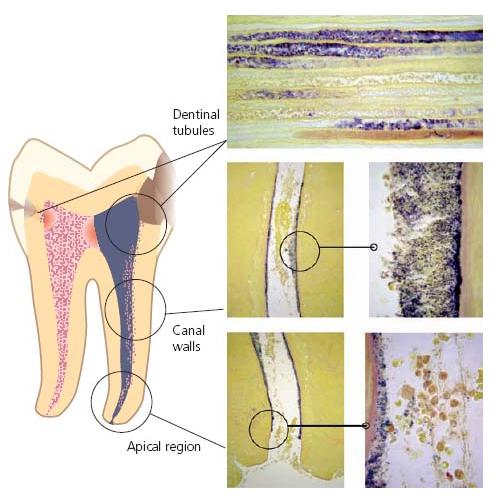
When bacterial cells become densely packed and embedded in an extracellular matrix of polymers of host and microbial origin, the term microbial biofilm is used. Bacteria residing in such microbial communities have gained a great deal of interest in recent years because infections, including those of root canals, often involve microbial biofilms. While planktonic microorganisms can be easily eliminated, there is a major challenge for root canal therapy to combat bacteria firmly attached to the root canal walls. This is especially so when microorganisms are in all areas of the canal space as well as in the dentinal tubules (Fig. 6.2) (53).
Microbial biofilms are ubiquitous in nature in all situations where natural liquid and microorganisms come in contact with surfaces (16). Dental plaque is one of the most studied biofilms and most members of the oral microflora contribute to the extensive biofilm formation on tooth surfaces. What is known about this particular biofilm also has implications for biofilms in the root canal environment (Fig. 6.6).
Independent of milieu, the development of biofilms seems to follow essentially the same sequence of events, starting with the adsorption of macromolecules to which microorganisms attach. Coadhesion of other microorganisms subsequently occurs and their attachment may be strengthened through polymer production and unfolding of cell surface structures. The multiplication and metabolism of adhering microorganisms ultimately result in the development of a structurally organized microbial community that is in a state of balance with the local environment (7, 37). Many natural biofilms have a highly diverse microflora with the individual species also organized functionally. When mature biofilms are viewed using non-invasive and non-destructive techniques (e.g. confocal laser scanning microscopy, CLSM), significant structural features observed include microcolonies of bacteria embedded in an extracellular matrix with channels or pores traversing the depth of the biofilm (4, 15).
Biofilms in root canals
The most direct evidence for biofilms in root canals comes from light microscopy, transmission electron microscopy and scanning electron microscopy studies in situ of extracted teeth with apical periodontitis (33, 47). Biofilms, most often composed of several morphotypes, grow in multilayers or as aggregates on the dentin walls of the root canal (Figs 6.3 and 6.4) or as dense aggregates in the necrotic tissue (Fig. 6.5). The amorphous material filling the interbacterial spaces in biofilms has been interpreted as being an extracellular matrix of bacterial origin. In many cases, the spatial organization described for root canal biofilms resembles that reported for dental biofilms (32). Hence, palisade structures of filaments and chains of cocci perpendicular to the canal wall (46), or corn-coblike structures of cocci attached to filaments (44) have been described.
Fig. 6.2 Microbial biofilms in dentinal tubules and on dentinal walls of the root canal. (Histological specimen courtesy of Dr D. Ricucci.)
Because of difficulty in accessing naturally occurring root canal biofilms with CLSM, information on their detailed architecture is limited. However, an open architecture is likely to exist enabling molecules, such as nutrients and microbial products, to move readily in and out of the biofilms.
Extraradicular colonization
The necrotic tissue of a non-vital pulp is the major location of microorganisms causing apical periodontitis, but the lesion per se may also harbor microorganisms. They may be attached to the root tip, or occur either as free-floating single cells or in clusters.
Indeed, multispecies biofilms have been described on the outer root surface of teeth adjacent to the apical foramen (30, 33). Such aggregations have been composed of various microbial forms, even including yeasts (46, 65) (Fig. 6.7). These colonizers are of particular significance from a treatment aspect in that an orthograde approach for endodontic treatment will not be able to eliminate the infection. However, it is not clear how common such extraradicular microbial aggregations are. They have been demonstrated mainly in cases with acute symptoms with and without sinus tracts (fistulae), or in cases not responding to endodontic treatment.
Microorganisms continuously detach from the surface of biofilms for colonization of other sites. In endodontic infections, released organisms may exit the apical foramen and may, at least temporarily, be present in the periapical tissue lesion per se, either in a free-floating state or inside phagocytes or both. This is normally the case in apical abscesses, which may harbor a variety of microbial forms.
Although microorganisms, including rods, spirochetes and cocci, have also been identified in asymptomatic periapical lesions of root filled teeth (59), well-organized tissue lesions, so-called apical granulomas (Chapter 7), are not normally found to be infected. An exception to this rule is the occasional finding of typical actinomyces-containing colonies (48). Radicular cysts may also harbor clusters of microorganisms (49).
Ecological determinants for microbial growth in root canals
As is the case for dental plaque (for review, see Ref. 38), adherence and coadherence are key ecological determinants for the survival and persistence of oral bacteria in the root canal environment. Low oxygen tension and the level of nutrients available from the host are other important ecological factors determining the success or failure of microorganisms entering the root canal to survive and grow (Core concept 6.1). The root canal biofilms contain multiple species in close physical contact, and this increases the probability of both synergistic and antagonistic interactions between microbial cells. With time, a multispecies microbial community is established and microbial interactions eventually give stability to the community. This mode of colonization gives rise to a chemical heterogeneity in the biofilm environment, which encourages phenotypic diversity. It also enhances the ability of the microorganisms to resist environmentally derived stressful conditions (see Advanced concept 6.1). Once a stable biofilm is established, disruption of the habitat, such as application of mechanical forces and antiseptics, must occur before the microbial community is significantly affected.
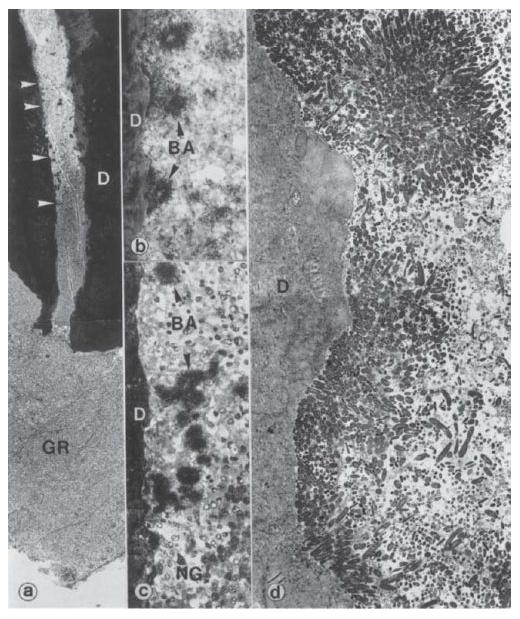
Fig. 6.3 Microbial biofilm in the apical part of a human tooth with apical periodonti-tis (GR). The areas between the upper two and the lower two arrowheads in (a) are magnified in (b) and (c), respectively. Note the dense bacterial aggregates (BA) sticking (in b) to the dentinal (D) wall and also remaining suspended among neutro-philic granulocytes (NG) in the fluid phase of the root canal content (in c). The neutrophilic granulocytes appear to form a defensive wall, against the advancing bacterial front. A transmission electron microscopic view (d) of the pulpodentinal interphase shows bacterial condensation on the surface of the dentinal wall, forming a thick layered plaque. Magnification: (a) ×46; (b) ×600; (c) ×370; (d) ×2350. (From Nair (49).)
Fig. 6.4 A community consisting of cocci and rods in an ecological niche on the root canal wall. The aggregated bacteria also show some penetration into the dentinal tubules. Scanning electron microscopy, magnification ×5000. (From Sen et al. (53).)
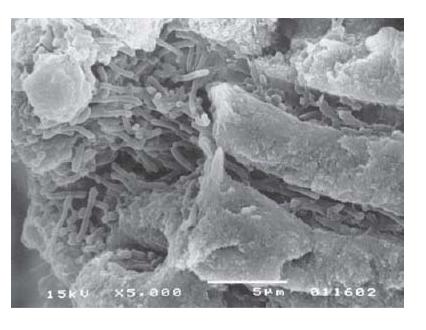
Fig. 6.5 Microbial biofilm in the apical region of a root canal. (a) Radiograph showing a lower incisor with apical periodontitis. (b) Scanning electron microscopic view of the apical region of the root canal. Scale bar: 500 μm. The delimited area is zoomed in in (c) where bacterial biofilm formation within the necrotized tissues is apparent. Scale bar: 5 μm. (Courtesy of Dr D. Jaramillo and Dr C. Schaudinn.)
Fig. 6.6 Schematic illustration of the development of biofilms on root canal tissues. Adherence and coadherence of microorganisms are followed by division and growth which are dependent on nutrients from the environment. The final composition reflects the outcome of metabolic and molecular interactions between members of the microbial community of the biofilm. (Adapted from the model designed by Dr G. Bowden.)
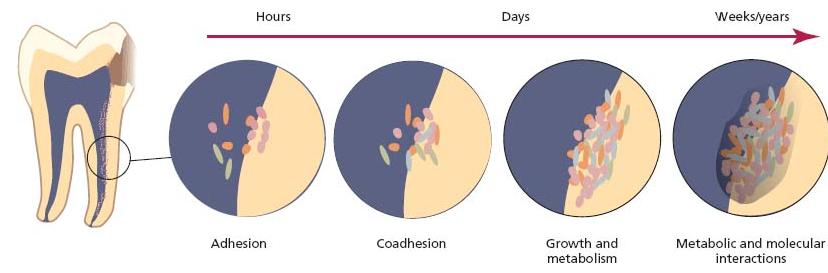
- Adhesion to root canal tissues
- Coaggregation of populations
- Low oxygen concentration and redox potential
- Nutrition
- Microbial interactions
- Endodontic treatment
Nutrition
Microorganisms entering the necrotic tissue in root canals encounter suitable conditions for growth. The necrotic tissue, tissue fluid and inflammatory exudates from the apical tissue supply the basic requirements for carbon, nitrogen, salts and energy as well as the special requirements for amino acids, nucleotides, vitamins and hemin (62). Nutrients can be derived directly from these sources but also from degradation of macromolecules such as proteins and glycoproteins, a process that requires the concerted action of enzymes released from different species in the microbial community. The resulting carbohydrates, small peptides and amino acids then serve as nutrients and an energy source for many inhabitants of the biofilm. The biofilm organisms may also benefit from intermicrobial food chains where metabolic end-products (e.g. NH3, CO2 and organic acids) of one species serve as nutrients for others. Owing to the small amount of carbohydrate available directly or liberated from degradation of glycoproteins, growth of microorganisms requiring carbohydrate for energy is limited, whereas proteolytic and amino-acid degrading microorganisms are favored. This is reflected in the high proportion of proteolytic bacteria present in the endodontic microbiota. The nutrient conditions in the root canal appear, in many ways, to be similar to those experienced by subgingival dental biofilms (for a review, see Ref. 58). In clinical situations where the necrotic tissues in root canals have been removed and the apical inflammatory process controlled, nutrients will be scarce. In nature, many microorganisms can survive such hostile environments by inducing a starvation response. While information on the starvation response in root canal bacteria is limited, microorganisms are likely to regulate their metabolic balance away from multiplication, towards the acquisition of energy for survival (21). It is quite possible that the adoption of a non-growing state could be an important mechanism for survival in nutrient-deprived environments of the root canal (12).
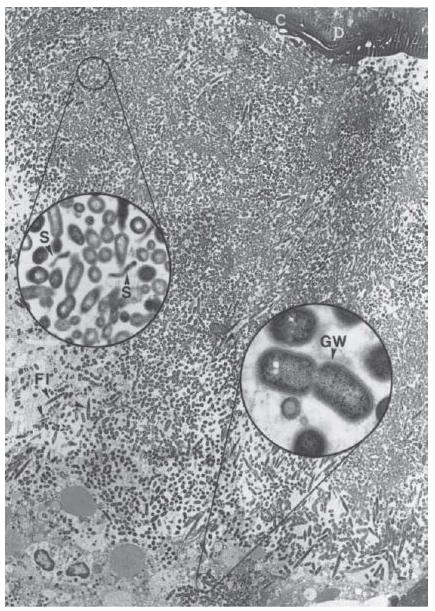
Fig. 6.7 Microbial biofilm at the periapex of a human tooth with acute apical periodontitis. Note the mixed bacterial flora consisting of numerous dividing cocci, rods (lower inset), filaments (FI) and spirochetes (S, upper inset). Rods often reveal a Gram-negative cell wall (GW, lower inset). C = cementum; D = dentin. Magnification: ×2680; upper inset: ×19 200; lower inset: ×36 400. (From Nair (49).)
Redox potentia/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses