Chapter 40 Extraoral Autogenous Donor Bone Grafts for Endosteal Implants: Ilium and Tibia
In ridges with abundant bone, all factors converge to provide an ideal long-term prognosis for the implant-supported prosthesis, including favorable ridge relationships. These conditions allow long and wide endosteal implants in adequate numbers, favorable crown/implant ratio, adequate bone density, and favorable orientation of occlusal forces toward the support system. Patients with these advantages usually have less-demanding needs than when unfavorable factors include advanced bone atrophy, as well as the dire need for prosthetic support and retention. When confronted with cases of extreme alveolar and basal bone resorption or discontinuities, most conventional treatment modalities fall short of the intended result. Instead, autogenous bone grafts from extraoral origin, in conjunction with implants, are often indicated to restore these patients.1–16 In addition, patients with moderate resorptive conditions and ideal prosthetic goals may also require augmentation.
HISTORY
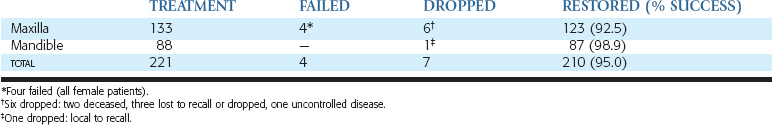
Throughout the evolution of oral implantology, several approaches have been proposed to address the problems of the severely resorbed jaw. The lack of support for traditional endosteal implant designs has led to the development of the subperiosteal implant, resting on the remaining bone of the ramus and symphysis in the mandible.17–18 The endosteal ramus frame implant design also takes advantage of these areas.19 In the maxilla, extension to the pterygoid plates, maxillary tuberosity, and lateral aspect of the zygomatic process are attempted with endosteal and subperiosteal implants.20,21 However, the extremely atrophic conditions and the resultant poor biomechanics of the implant system have led to many complications (Figure 40-1). The violation of fundamental prosthodontic principles in the final restoration, as insufficient implant support (in size, number, and location) and excessive number of pontics, has encouraged the development of more predictable procedures.
The concept of jaw reconstruction with varied autogenous and alloplastic materials has been used extensively for many years in craniofacial reconstruction.22–25 However, jaw reconstruction with autogenous bone alone has been characterized by rapid, advanced bone resorption during the 3 to 5 years after the procedure.26–29 In addition, augmentation of atrophic ridges for conventional complete dentures still does not return a patient to normal mastication and comfort (Figure 40-2). On the other hand, reports of implants in conjunction with autogenous grafts have been reported in the literature, with improved success in relation to prosthetic support and long-term maintenance of the graft. The implants stimulate and maintain the grafted bone and prevent the rapid bone resorption found with autogenous grafts for complete denture support.1,16,30,31
When the placement of endosteal implants is desired and advantageous for prosthodontic support, it is beneficial to reconstruct the anatomy of the atrophic jaw with bone or a material that will resorb and be replaced by living bone. The posterior maxilla can be augmented predictably by sinus grafting to permit the placement of endosteal implants, using synthetic bone substitutes, combined with demineralized freeze-dried bone (DFDB) and intraorally harvested autogenous bone.32,33 No other region of the mouth permits as predictable augmentation for endosteal implants in the severe atrophic state. The subantral (SA) area created for sinus grafts presents many features that are favorable for bone formation. The graft is surrounded by bony walls in a well-vascularized region and protected from loads during healing. Other areas of the mouth that require ridge augmentation more often require onlay autogenous bone grafts to predictably gain bone volume. As a consequence, in most regions of the mouth, the treatment of choice for moderate to severe atrophic conditions is reconstruction with autogenous bone grafting, followed by oral rehabilitation with implant-supported prostheses.
Treatment Planning
Indications
Indications for autogenous grafts for the placement of endosteal implants are presented using the Misch-Judy available bone and Misch prosthetic options classifications.33a,33b In addition, bone Division E has been added to the original four divisions to encompass those conditions in which trauma, pathology, surgery, or genetics have resulted in a discontinuity defect of the jaw.
General Considerations
Autogenous bone grafts are osteogenic and able to form bone in the absence of undifferentiated mesenchymal cells. In addition, the autograft may form bone by osteoinduction, osteoconduction, and a barrier membrane approach. In addition, it appears that no age-related change exists in the viability of bone-derived cells.34 Fresh, autogenous, cancellous bone provides the survival of the maximum number of transplanted bone and pluripotential cells of the marrow.23 For large grafts, it is usually harvested from the ilium, which presents a high population of pluripotential cells and can deliver the greatest amount of trabecular bone.35–37 Proponents of the use of solely trabecular bone argue that when harvested from iliac crest, it allows implant placement earlier.38 Fonseca and Davis37 recommended the addition of chips from the lateral cortex to increase the bone morphogenetic protein (BMP) concentration. The addition of platelet-rich plasma (PRP)35,39,40 over the grafted bone attempts to further stimulate the second phase of bone formation and may enhance and stimulate remodeling and incorporation of the graft. However, trabecular grafts are usually maintained in place by the use of cribs.36 More importantly, the resorption rate is faster than the bone formation rate, and the volume of bone obtained is highly variable. In addition, the quality of bone formed is often poorer compared with other graft options.
An advantage of a trabecular graft is the solid block form, which permits contouring and adaptation of the graft to the recipient bed anatomy.41 The block graft maintains greater volume of bone compared with particulate onlay grafts, because resorption of the cortical bone is delayed and rigid fixation of the block to the atrophic ridge is possible. The cortical bone on the outside of the graft also acts like a barrier membrane similar to membranes used in guided bone regeneration (GBR).42 The fibrous tissue is prevented from invading the graft site, and this provides additional time for angiogenesis and new bone formation below the cortical layer. The rigidity and stability of the graft can be achieved by the use of fixation screws to stabilize the graft, eliminating the need for wiring, pins, or the use of a crib.2,3 The quality of the bone is greater and increases strength, modulus of elasticity, and initial bone-implant contact percent (BIC). A review of the literature of bone grafts and the placement of implants reported similar success rates for block and particulate grafts to the maxilla and mandible,6 and both procedures are widely reported.
ILIAC CREST CORTICAL AND TRABECULAR BLOCK GRAFTS
According to several publications, autogenous bone harvested from the ilium is the donor site of choice for large-defect bone grafts to the jaws.1–16,36,43–53 The primary advantage of the iliac crest graft is its large volume. The outer portion of the graft may be primarily cortical, with abundant trabecular bone underneath. The volume of bone harvested permits shaping of two thirds of a mandible or maxilla or filling of larger bony voids.
Relative ease of access and harvesting from the ilium have made it a safe and well-accepted procedure. However, rapid bone resorption of 30% to 90% of the iliac crest bone grafts has been reported when conventional dentures are placed on top of the reconstruction.29 As a consequence, other treatment options have often been considered, such as zygomatic and pterygoid implants in the maxilla or 7- to 9-mm implants in an anterior mandible. However, the placement of implants into the grafted bone has dramatically modified this resorption rate. Once the implants are placed and in function, the rate of bone resorption is similar to host bone of similar quantity and density in similar conditions.5,53–55
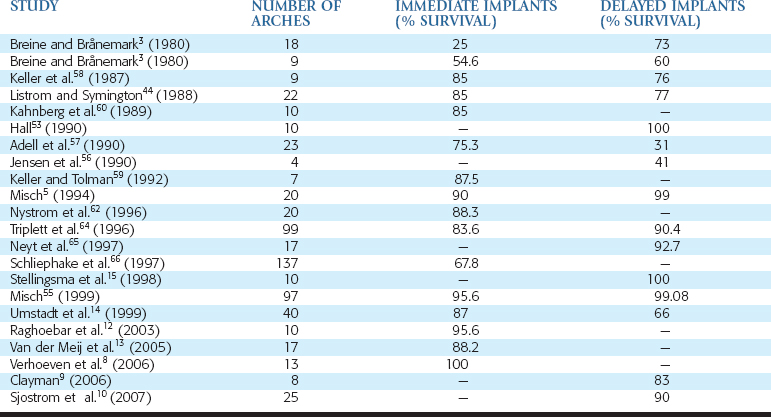
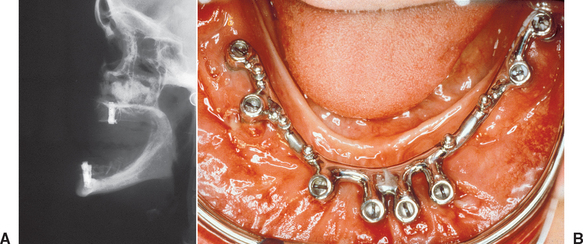
In 1972, Kratochvil and Boyne30 originally reported the iliac crest bone graft procedure with the concomitant use of implants with subperiosteal implants (Figure 40-3). In the past decades, an increasing number of reports have discussed concomitant use of grafted bone from the iliac crest with endosteal root form implants placed simultaneously or in a second step.1–16,53–75 However, the reported success rates of autogenous bone grafts and dental implants are variable (Table 40-1).
In the early years of reports, implant failure was higher in grafted bone than native host bone. Breine and Bränemark3 placed implants in 18 atrophic jaws in conjunction with autogenous trabecular bone. Only 25% of the 129 implants remained integrated in 14 atrophic maxillae and four mandibles. Most jaws required additional implants, and 20% of the maxillary jaws never achieved permanent bridge stability. Additional implants placed after graft maturity had 73% survival. In 1987, Keller et al.58 reported on five patients with 28 implants and bone grafting, with four failing to fixate (85% survival) and four patients with 76% survival (16 of 21 implants) when placed after Le Fort I osteotomy and grafts. They later reported on these patients and 20 additional grafted maxillae after a 2-year evaluation, with an overall implant survival of 77.4%. Keller and Tolman59 reported on seven mandibles with iliac crest grafts and 32 simultaneous implant placements, resulting in 87.5% survival rate. In 1988, Listrom and Symington44 inserted implants in 12 grafted arches, with 85% initial implant survival, as well as a secondary procedure in 10 patients with 43 implants and 77% implant survival. Kahnberg et al.60 reported on 10 patients in 1989 having received 57 implants simultaneously, with a graft from the iliac crest and eight implants lost (85% survival). Complications included graft exposure in three patients (30%). The use of a splint to protect the graft was reported to significantly decrease this complication.
Results by the same authors were updated in 1995, with 20 arches grafted with simultaneous implant placement and an 88.3% survival rate.54 In 1997, Schliephake et al.66 reported on the 5-year follow-up of 137 grafted atrophic maxillae and a cumulative survival rate of 67.8% for implants placed at the time of grafting.
In 1990, Jensen et al.56 also reported problems with iliac bone grafts and stated the procedure should still be considered in an evaluation stage. Twenty-nine implants were placed in four patients who received iliac crest grafts. Twelve remained fixated and in function, or 41% implant survival. In a study of 23 consecutive grafted maxillae over a 10-year period, Adell et al.57 reported on 124 fixtures placed simultaneously with the grafts and 16 implants added in a second stage. The survival rate of fixtures placed with the graft was 75.3% at 4 years after grafting. However, 16 implants placed at a secondary procedure resulted in nine implant failures (31% implant survival).
On the other hand, several reports in the literature indicate implant survival in grafted bone is similar to native bone. Hall53 reported 100% implant success after graft maturity in 10 patients and also reported bone loss similar to nongrafted bone sites where implants were inserted. Tidwell et al.63 reported on combined sinus and iliac onlay grafts in 48 maxillary patients and found 6.4% implant failure in the sinus and 7.8% implant failure in the anterior region. In 1996, Triplett et al.64 reported on 129 grafts in 99 patients (including ilium, cranium, and symphyseal grafts). Survival rates for the implants placed at the time of grafting were 83.6% and 90.4% for implants placed in a delayed approach. In 2003, Raghoebar et al.12 reported on the early loading of 68 implants inserted in a single-stage approach, with iliac crest grafts to 10 maxillae and a 95.6% survival rate after 1 year.
The ideal timing for implant placement in autogenous bone grafts from extraoral origin remains a controversial issue. In 1997, Neyt et al.65 reported on 17 atrophic maxillae restored with sinus and iliac crest grafts and implants and an overall 92.7% survival rate. All implants were placed in a delayed fashion. In 1999, Umstadt et al.14 performed 40 iliac crest grafts in a prospective study of 176 implants and reported 87% and 66% survival rate for implants placed in a immediate and delayed fashion, respectively. In 1998, Stellingsma et al.15 reported 100% success for 40 implants placed in a delayed fashion in 10 augmented mandibles of female patients.
In 2005, Van der Meij et al.13 also selected an immediate implant placement approach for 17 atrophic mandibles augmented with the iliac crest and restored with two implant overdentures (34 implants). The average length of the study was 4.3 years, with an 88.2% implant success rate. In 2006, Verhoeven et al.8 reported 100% implant survival rate when placed at the same time as the bone graft in 13 atrophic mandibles, after 10 years. However, 51% of the bone graft had resorbed, mostly during the first year after the bone graft. In 2006, Clayman9 reported on eight grafted maxillae with delayed placement of 34 implants, with an 83% success rate. Sjostrom et al.10 elected to place all implants in a delayed fashion. They grafted 25 maxillae that were restored with 192 implants. At the 3-year follow-up, 90% were successful with stable bone levels.
In conclusion, recommendations in the literature range from immediate placement to delays of 4 to 12 months postoperatively. Requirements for the immediate-placement success include the anchorage of immediate implants in native bone, with a minimum of 1.5-mm thickness of graft bone covering the implant. Several other reports underline that implants anchored in graft alone fail.68–70 Graft maturation is the primary advantage of delayed implant placement, along with the possibility to address eventual postoperative complications and a more precise placement of the implants for the intended prosthesis. Timing of implant placement should, however, attempt to take advantage of the bone-preserving stimulus.44,71–73 It is also suggested that the implant and graft success may be different whether a maxilla or mandible is the host site.
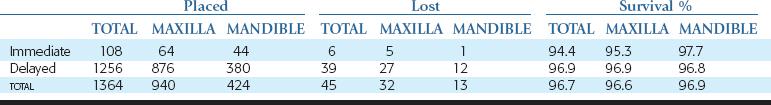
A retrospective case series study was published in 1994 by Misch and updated in 1999.55 This report is further updated in this chapter. Patients received iliac crest block grafts for implant-supported prostheses from 1984 to 2005 and were monitored until 2007 (Table 40-2). A total of 182 patients and 221 arches received autogenous bone grafts from the iliac crest to the maxilla (133) and mandible (88). Participants included 146 women and 36 men in the study group. Thirty-three women and six men received grafts in both the maxilla and the mandible. Six arches in the maxilla were lost to recall and dropped (two patients died before treatment was completed, three patients elected not to continue treatment for economic and personal reasons, and one patient developed an uncontrolled disease and elected not to continue treatment [not reentered]). One arch in the mandible was dropped because the patient elected not to continue treatment for economic reasons (same patient as maxilla). These seven arches were dropped from the study.
| PATIENTS | ARCHES | |
|---|---|---|
| Male patients | 36 | 42 |
| Female patients | 146 | 179 |
| TOTAL | 182 | 221 |
A total of four arches failed (all female patients and all maxillae). Therefore a total of 210 arches were restored (123 maxillae, 87 mandibles), with a total success rate of the graft of 95% (92.5% and 98.9% for maxillae and mandibles, respectively) (Table 40-3).
Ninety-one implants were placed to stabilize the block graft, of which 44 were in the mandible and 64 in the maxilla (Table 40-4). Six months later, the implants were evaluated by direct observation for rigid fixation and quantity of bone around the implant. Three implants were mobile, and three other implants exhibited rigid fixation; however, two had poor placement, and one had more than one third of crestal bone loss. Therefore six implants were removed (five of the six were in the maxilla), with 97.7% of mandibular immediate implants placed maintained, whereas 92.2% of maxillary immediate implants were maintained (overall survival 94.4%). After 1993, no implants were placed concomitantly with the grafts to fixate the graft. In some selected cases, however, some were placed in the tuberosity or vomer to support the transitional prosthesis.
Five to 8 months after the autogenous bone graft, 1256 endosteal implants were placed in a delayed fashion. The maxillae received 876 implants, and the mandibles received 380 endosteal implants. Thirty-seven implants did not achieve rigid fixation and were removed at the stage II (uncovery) surgery. Two additional implants were lost after 2 years in function in the posterior maxilla. The survival of the implants placed in a delayed fashion in autogenous bone graft sites was 96.9% in the maxilla and 96.8% in the mandible, with an overall survival rate of 96.9% (see Table 40-2).
Several authors have advocated the placement of implants after graft bone maturation for 5 to 10 months. A delayed implant placement after graft maturity of 5 to 10 months is also strongly encouraged for several reasons. Incision line opening is a common complication with larger bone grafts. Implants exposed along with the surrounding graft are more likely to fail or exhibit crestal bone loss. In addition, because the first 5 to 10 months are a primary time of consolidation and/or remodeling of the graft and modeling of new bone, the contours and volumes of the bone block exhibit the greatest changes.61,74,75 Functional loading of the implants allows this resorption to fall to the value of physiologic involution. The delayed approach permits the placement of implants in optimized bone volume, and it even allows regrafting in regions compromised after initial graft healing. Prosthetic and surgical templates may be used with greater accuracy for delayed implant placement, with precise information on the bony topography for surgical template fabrication. As a result, implants are positioned more ideally for the intended prosthesis.
Procedure
Maxillary Bone Grafts
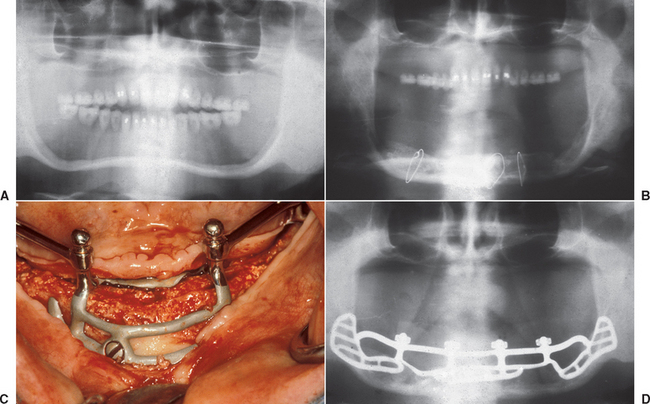
The iliac crest graft technique to the maxillae has evolved over the last 25 years. Different approaches such as onlay grafting or intrapositional graft with Le Fort I osteotomies have been proposed. Originally, implants were often placed at the same time as the bone graft and positioned anterior to the maxillary sinuses.3,44,45,56,57 Because the maxilla resorbs to the medial and the mandible resorbs toward the facial as basal bone remains, implants placed at the time of the bone graft are too lingual in the maxilla and too facial in the mandible (Figure 40-4). Poor implant position, increased risk of implants in compromised areas of incision line opening, poor anteroposterior (A-P) implant distance, and less-controlled crestal bone loss during bone revascularization suggested several modifications of the technique.
Another main surgical advantage of early sinus grafting is the opportunity to enhance the soft tissue quality and quantity in the anterior region for primary coverage of the onlay graft. A transitional augmentation may be performed in the premaxilla. A full-thickness incision is made 5 mm to the palatal aspect of the crest from canine to canine. A mucoperiosteal flap exposes the facial region of the ridge. A Vicryl-encapsulated dense hydroxyapatite (HA) (PermaRidge) or AlloDerm acellular tissue graft is placed in the premaxilla region. The mesh of dense HA or acellular tissue acts as a transitional augmentation during soft tissue healing. At the iliac crest graft surgery, the HA “sausage” is removed before positioning the iliac block of bone. The primary advantage is the improved tissue thickness available to allow primary closure over the graft without tension.
SURGICAL APPROACH: HARVEST OF THE ILIAC CREST TRICORTICAL BLOCK
Anterior Iliac Crest Graft
The subcrestal window technique has been described by Behairy and Al-Sebai.76 This provides a biocortical full-thickness graft while sparing an 8- to 10-mm bridge of iliac crest proximally. However, the harvest of the tricortical bone block is primarily used because of the excellent ridge of cortical bone provided by the crest. The procedure for harvest is described along with the reconstruction technique to prevent cosmetic deformity. This technique also limits hematoma formation caused by exposed cancellous bone. Herniation of abdominal contents is a well-known77–82 complication of full-thickness iliac crest bone grafting. The technique described may limit this complication.83 With proper technique, the harvest of tricortical bone graft from the anterior iliac crest can be performed with good results.
Procedure for Harvest of Iliac Crest
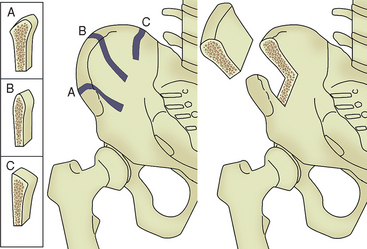
Place the patient supine and administer general anesthetic, with placement of a nasotracheal airway to allow free access to the oropharynx. The oral surgery is typically performed after harvest because of space constraints around the patient. If only a one-sided harvest is planned, then the surgeon should place a bump under that side. In selecting the side for harvest, one consideration is the side preferred for sleeping. If bone is to be harvested bilaterally, then no bump is used. Primarily, three different harvest sites of the ilium exist (Figure 40-5). The anterior iliac crest is preferred, because the posterior harvest is usually limited to one or two cortical plates and a tricortical bone harvest is desired. In addition, bone width at the crest of the ilium is often greater in the anterior region compared with the midcrestal anatomy. A 7- to 8-cm transverse incision is made either proximal or distal to the iliac crest. An incision directly over prominence of the iliac crest is avoided84 because of likely irritation from clothing or seat belts. The incision must remain at least 2 cm lateral to the anterior superior iliac spine (ASIS) to avoid damage to the lateral femoral cutaneous nerve (LFCN), which can rarely course through this area. Electrocautery is used for hemostasis and to dissect through the subcutaneous tissues to the fascia overlying the ilium.
Beginning at least 2 cm lateral to the ASIS, the relatively avascular interval between the abdominal musculature superiorly and the abductor musculature inferiorly is identified and incised with electrocautery directly over the ilium.85 Subperiosteal dissection by means of electrocautery and a Cobb elevator is used to expose the inner and outer tables of the iliac crest, clearing away the iliacus muscle medially and gluteal muscle at the outer table. It is imperative to remain in a subperiosteal plane during this exposure to avoid damaging neurovascular structures on the inner table. This minimizes muscular bleeding and thus hematoma formation. Staying within this plane also decreases postoperative pain and creates a thick periosteal and facial layer for adequate repair. A self-retaining retractor provides excellent visualization at this point.
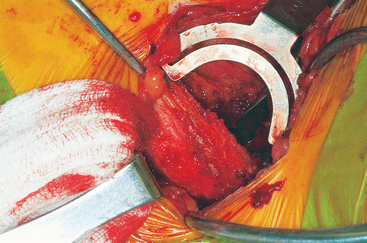
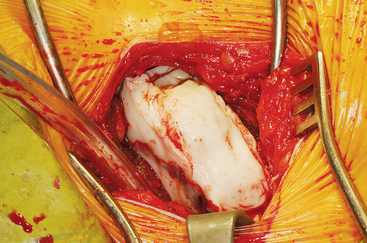
The ASIS is located and the iliac crest is cut sagittal at least 3 cm lateral, being careful to avoid wandering anteromedially. Attention to these details lessens the likelihood of ASIS avulsion by sartorius and tensor fascia latae muscles. The cut is made using the oscillating saw. The oscillating saw is preferred for sagittal cut as a result of biomechanical studies, which have shown that cutting the iliac crest with the osteotome has the effect of weakening graft strength.86 A sizing guide is then taken (Figure 40-6), as determined by the oral surgeon preoperatively. It is placed on the inner table, and the size is marked directly onto the ilium. Care is taken to obtain a graft to match the sizing guide, because an inadequate graft lengthens the oral procedure considerably by requiring piecemeal reconstruction. A second sagittal cut is made with the oscillating saw. The area of the ilium where the bone thins considerably is located, and the bone is carefully osteotomized. This bone is then transferred to a basin with sterile saline until the oral surgeon begins contouring.
Technique for Reconstruction of the Iliac Crest
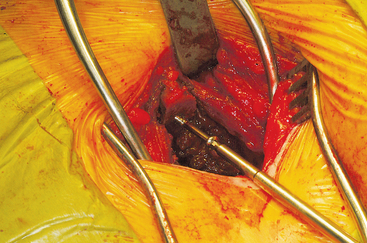
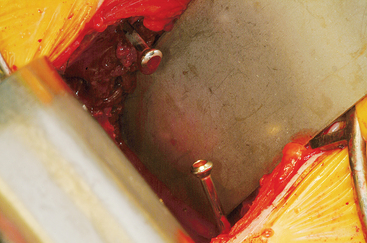
The wound is well irrigated with an antibiotic solution and suctioned dry. The pelvis is then inspected for fracture. A 0.062-inch unthreaded Kirschner wire is driven into one of the cut edges of the cancellous bone left exposed at the donor site. The Kirschner wire is measured, and a 3.5-mm cannulated cancellous screw is chosen so that about one third to half of the screw’s length is left exposed, at least 1.0 to 1.5 cm (Figures 40-7 and 40-8). This cannulated screw is then driven over the Kirschner wire in usual fashion, and the Kirschner wire removed. Methylmethacrylate cement is mixed. Using the exact same procedure, a second screw is placed opposite the first (Figure 40-9). These screws are placed to act as rebar to stay the cement once it is placed.
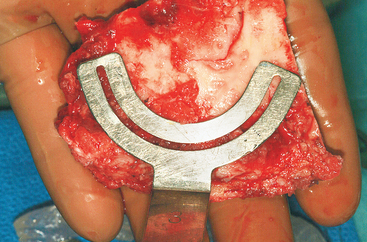
Figure 40-7 A measuring guide is used to determine and verify sufficient volume of the harvested block.
Malleable retractors are now placed at the inner and outer tables, flush with the bone, to provide containment for the cement. Once the cement reaches the consistency of putty, an appropriate amount is placed at the reconstruction site so that it mimics the bone block harvested. Great care is taken with the use of the malleable retractors and large osteotomes, if necessary, to contain the cement (Figure 40-10). As the cement hardens, the retractors are moved slightly to prevent adherence of the cement. The superior aspect of the cement is smoothed with the fingers so that the bone-cement interface is smooth and contoured to the normal anatomy. More cement is added if necessary (Figure 40-11). The wound is irrigated during hardening to prevent thermal damage. Once the cement is hardened, retractors are removed and the reconstruction is inspected for loose cement. Sharp edges are trimmed with a rongeur (Figure 40-12).
Neurovascular Structures
The LFCN typically crosses the anterior surface of the iliacus muscle before passing into the thigh, near the ASIS. Normally the nerve passes beneath the inguinal ligament and sartorius muscle; however, in as many as 10% of cases, it may pass over the iliac crest at a point as much as 2 cm lateral to the ASIS.87,88 The technique, as described, prevents damage to the LFCM.
At the level of the anterior iliac crest, the ilioinguinal nerve passes over the transversus abdominis and internal oblique muscles. It then courses under the external oblique muscle, entering the inguinal canal to pass distally, supplying sensation to the genitalia and nearby skin. Great care should be taken to approach the inner table in the subperiosteal fashion and careful attention given when retracting or using the oscillating saw.89
The femoral nerve is also at risk when harvesting bone from the inner table. Located more distally than the ilioinguinal nerve, it is found in the iliac fossa as it courses over the iliacus muscle, entering the thigh below the inguinal ligament.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses