Biomaterials for Dental Implants
Jack E. Lemons, Francine Misch-Dietsh, Michael S. McCracken
Compatibility of Surgical Biomaterials and the Role of Synthetic Materials
The biocompatibility profiles of synthetic substances (biomaterials) used for the replacement or augmentation of biological tissues have always been a critical concern within the health care disciplines. Special circumstances are associated with dental implant prosthetic reconstruction of the oral-maxillofacial areas because the devices extend from the mouth, across the protective epithelial zones, and onto or into the underlying bone. The functional aspects of use also include the transfer of force from the occlusal surfaces of the teeth through the crown and bridge and neck-connector region of the implant into the implant for interfacial transfer to the supporting soft and hard tissues. This situation represents a very complex series of chemical and mechanical environmental conditions.
This most critical aspect of biocompatibility is, of course, dependent on the basic bulk and surface properties of the biomaterial. All aspects of basic manufacturing, finishing, packaging and delivering, sterilizing, and placing (including surgical placement) must be adequately controlled to ensure clean and nontraumatizing conditions. The importance of these considerations has been reemphasized through the concept and practice of osseointegration of endosteal root form implant systems.
The disciplines of biomaterials and biomechanics are complementary to the understanding of device-based function. The physical, mechanical, chemical, and electrical properties of the basic material components must always be fully evaluated for any biomaterial application because these properties provide key inputs into the interrelated biomechanical and biological analyses of function. It is important to separate the roles of macroscopic implant shape from the microscopic transfer of stress and strain along biomaterial–tissue interfaces. The macroscopic distribution of mechanical stress and strain is predominantly controlled by the shape and form of the implant device. One important material property related to design (shape and form) optimization is the elastic strain (one component of the elastic modulus) of the material.
The localized microscopic strain distribution is controlled more by the basic properties of the biomaterial (e.g., surface chemistry, microtopography, modulus of elasticity) and by whether the biomaterial surface is attached to the adjacent tissues. Engineering analyses of implant systems include optimization considerations related both to the design and to the biomaterial used for construction. Therefore, the desire to positively influence tissue responses and to minimize biodegradation often places restrictions on which materials can be safely used within the oral and tissue environments. Designs are often evolved for specific biomaterials because of the imposed environmental or restorative conditions.
Bulk Properties
History of Materials and Designs
Over the past several decades, definitions of material biocompatibilities have evolved and reflect an ever-changing opinion related to philosophies of surgical implant treatment. In general, the definition of biocompatibility has been given as an appropriate response to a material (biomaterial) within a device (design) for a specific clinical application.1 Metallic and nonmetallic implantable materials have been studied in the field of orthopedics since the turn of the twentieth century.2–7
In the 1960s, emphasis was placed on making the biomaterials more inert and chemically stable within biological environments. The high-purity ceramics of aluminum oxide (Al2O3), carbon, and carbon–silicon compounds and extra-low-interstitial (ELI) grade alloys are classic examples of these trends. In the 1970s, biocompatibility was defined in terms of minimal harm to the host or to the biomaterial. The importance of a stable interaction then moved into central focus for both the research and the clinical communities. In the 1980s, the focus transferred to bioactive substrates intended to positively influence tissue responses. In the past two decades, emphasis was on chemically and mechanically anisotropic substrates combined with growth (mitogenic) and inductive (morphogenic) substances. Today many biomaterials are being constituted, fabricated, and surface modified to directly influence short- and long-term tissue responses. Bioactive coatings on most classes of biomaterials have continued to evolve from human clinical trials to acceptable modalities of surface preparation, and research focus has shifted to combinations of active synthetic and biological implants.
Of interest, dental implants have significantly influenced these trends. In the 1960s, dental devices were recognized as being in a research and development phase, and critical longitudinal reviews of clinical applications were strongly recommended.8 During this time, longevity studies of various devices demonstrated that the longest duration of clinical applications were for orthopedic prostheses. In the 1980s, controlled clinical trials showed that dental implants provided functional longevities that exceeded most other types of functional tissue replacement modalities.9,10 Clearly, these clinical studies have strongly influenced both the research and development and the clinical application processes. At the present time, the exponential growth of implant use and related scientific reports support the views expressed by early visionaries several decades ago.
The evolution of any implant modality is a multipart story in which significant roles have been played by biomaterials; biomechanical analyses of designs, tissues, and function; wound healing along interfaces; surgical methods to minimize mechanical, chemical, and thermal trauma; prosthodontic and periodontal restorative and maintenance treatment modalities; and protocols for controlled multidisciplinary clinical trials. The interdependence of all phases of basic and applied research should be recognized. All interrelate and must evolve to provide a level of better understanding of the basic physical and biological phenomena associated with the implant systems before the longer clinical outcomes will be fully described.
Evaluations of endosteal and subperiosteal dental implants raise interesting questions with respect to the interrelationships between material and design selection. Opportunities exist to select a material from a number of systems, such as metals, ceramics, carbons, polymers, or composites. In addition, only the available anatomical dimensions and the requirement to attach some form of intraoral restorative device limit implant shape and form (design). Because of the wide range of biomaterial properties demonstrated by the classes of materials available, it is not advisable to fabricate any new implant design without a thorough biomechanical analysis. Another approach now often used is to determine a specific design based on clinical considerations and then to select the biomaterial of choice from computer-based analyses. The safety of these combinations can then be demonstrated through laboratory and animal investigations. Controlled clinical trials after prospective protocols, of course, provide the final evaluation for both safety and effectiveness. Long-term success is thus determined clinically in investigator follow-up studies and is clearly an area that should be emphasized for many available dental implant systems.
Research and Development
Basic studies within the physical and biological sciences have been supportive of the development of surgical implant systems. One example is the continued progress from materials that have been available for industrial applications to the new classes of composites that have evolved for biomedical applications. This same situation exists within a broad area (e.g., surface science and technology, mechanics and biomechanics of three-dimensional structures, pathways and processes of wound healing along biomaterial interfaces, and the description of the first biofilms that evolve on contact with blood or tissue fluids).11–14 The progressive move from materials to quantitatively characterized biomaterials has been extremely important to the biomedical applications of surgical implants. Dental implant investigations now play a leadership role within selected areas of this overall process, and all phases of medicine and dentistry should benefit.
Physical, Mechanical, and Chemical Requirements for Implant Materials
Physical and Mechanical Properties
Forces exerted on the implant material consist of tensile, compressive, and shear components. As for most materials, compressive strengths of implant materials are usually greater than their shear and tensile counterparts. A hypothesis that dental implants are less affected by alternating stresses than implants of the cardiovascular and locomotor systems because of the significantly lower number of loading cycles must be qualified because of the special concern that dental implants are considerably smaller in physical dimension. All fatigue failures obey mechanical laws correlating the dimensions of the material to the mechanical properties of said material.11,15 In addition, when present, parafunction (nocturnal or diurnal) can be greatly detrimental to longevity because of the mechanical properties, such as maximum yield strength, fatigue strength, creep deformability, ductility, and fracture. Limitations of the relevance of these properties are mainly caused by the variable shape and surface features of implant designs. A recurring problem exists between the mechanical strength and deformability of the material and the recipient bone. A different approach to match more closely the implanted material and hard tissue properties led to the experimentation of polymeric, carbonitic, and metallic materials of low modulus of elasticity.16,17
Because bone can modify its structure in response to forces exerted on it, implant materials and designs must be designed to account for the increased performance of the musculature and bone in jaws restored with implants. The upper stress limit decreases with an increasing number of loading cycles sometimes reaching the fatigue limit after 106 to 107 loading cycles.11,15,18 In other words, the higher the applied load, the higher the mechanical stress—and therefore the greater the possibility for exceeding the fatigue endurance limit of the material.
In general, the fatigue limit of metallic implant materials reaches approximately 50% of their ultimate tensile strength.11,18 However, this relationship is only applicable to metallic systems, and polymeric systems have no lower limit in terms of endurance fatigue strength. Ceramic materials are weak under shear forces because of the combination of fracture strength and no ductility, which can lead to brittle fracture. Metals can be heated for varying periods to influence properties, modified by the addition of alloying elements or altered by mechanical processing such as drawing, swagging, or forging followed by age or dispersion hardening until the strength and ductility of the processed material are optimized for the intended application.
The modifying elements in metallic systems may be metals or nonmetals. A general rule is that constitution or mechanical process hardening procedures result in an increased strength but also invariably correspond to a loss of ductility. This is especially relevant for dental implants. Most all consensus standards for metals (American Society for Testing and Material [ASTM], International Standardization Organization [ISO], American Dental Association [ADA]) require a minimum of 8% ductility to minimize brittle fractures. Mixed microstructural phase hardening of austenitic materials with nitrogen (e.g., stainless steels) and the increasing purity of the alloys seem most indicated to achieve maximum strength and maintain this high level of possible plastic deformation.1,15,19–23
Corrosion and Biodegradation
Corrosion is a special concern for metallic materials in dental implantology because implants protrude into the oral cavity, where electrolyte and oxygen compositions differ from those of tissue fluids. In addition, the pH can vary significantly in areas below plaque and within the oral cavity. This increases the range of pH that implants are exposed to in the oral cavity compared with specific sites in tissue.24–29 Plenk and Zitter15 state that galvanic corrosion (GC) could be greater for dental implants than for orthopedic implants. Galvanic processes depend on the passivity of oxide layers, which are characterized by a minimal dissolution rate and high regenerative power for metals such as titanium. The passive layer is only a few nanometers thick and usually composed of oxides or hydroxides of the metallic elements that have greatest affinity for oxygen. In reactive group metals such as titanium, niobium, zirconium, tantalum, and related alloys, the base materials determine the properties of the passive layer. The stability zones of the oxides of passivable elements cover the redox potentials and pH values typical of the oral environment. However, titanium, tantalum, and niobium oxides cover a markedly larger zone of environmental stability compared with chromium oxides.
The risk of mechanical degradation, such as scratching or fretting of implanted materials, combined with corrosion and release into bone and remote organs has been previously considered. For example, investigators such as Laing,30 Willert et al.,31 and Lemons,32,33 have extensively studied the corrosion of metallic implants. Steinemann34 and Fontana and Greene35 have presented many of the basic relationships specific to implant corrosion. Mears26 addressed concerns about GC and studied the local tissue response to stainless steel and cobalt–chromium–molybdenum (Co-Cr-Mo) and showed the release of metal ions in the tissues. Williams36 suggested that three types of corrosion were most relevant to dental implants: (1) stress corrosion cracking (SCC), (2) GC, and (3) fretting corrosion (FC).
Stress Corrosion Cracking
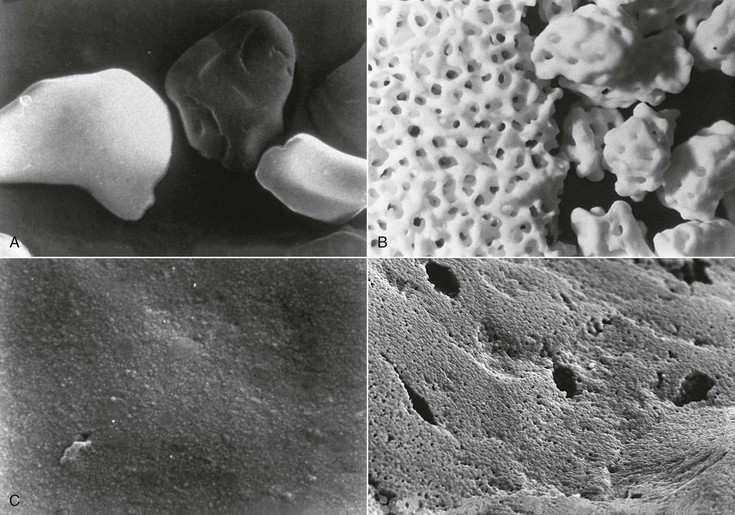
The combination of high magnitudes of applied mechanical stress plus simultaneous exposure to a corrosive environment can result in the failure of metallic materials by cracking, where neither condition alone would cause the failure. Williams36 presented this phenomenon of SCC in multicomponent orthopedic implants. Others hypothesized that it may be responsible for some implant failures in view of high concentrations of forces in the area of the abutment–implant body interface.37–39 Most traditional implant body designs under three-dimensional finite element stress analysis show a concentration of stresses at the crest of the bone support and cervical third of the implant. This tends to support potential SCC at the implant interface area (i.e., a transition zone for altered chemical and mechanical environmental conditions). This has also been described in terms of corrosion fatigue (i.e., cyclic load cycle failures accelerated by locally aggressive medium). In addition, nonpassive prosthetic superstructures may incorporate permanent stress, which strongly influences this phenomenon under loaded prostheses37,40,41 (Figure 4-1, A and B).
Galvanic corrosion occurs when two dissimilar metallic materials are in contact and are within an electrolyte resulting in current flowing between the two. The metallic materials with the dissimilar potentials can have their corrosion currents altered, thereby resulting in a greater corrosion rate (Figure 4-1, C). FC occurs when a micromotion and rubbing contact occurs within a corrosive environment (e.g., the perforation of the passive layers and shear-directed loading along adjacent contacting surfaces). The loss of any protective film can result in the acceleration of metallic ion loss. FC has been shown to occur along implant body–abutment–superstructure interfaces.
Normally, the passive oxide layers on metallic substrates dissolve at such slower rates that the resultant loss of mass is of no mechanical consequence to the implant. A more critical problem is the irreversible local perforation of the passive layer that chloride ions often cause, which may result in localized pitting corrosion. Such perforations can often be observed for iron–chromium–nickel–molybdenum (Fe-Cr-Ni-Mo) steels that contain an insufficient amount of the alloying elements stabilizing the passive layer (i.e., Cr and Mo) or local regions of implants that are subjected to abnormal environments. Even ceramic oxide materials are not fully degradation resistant. Corrosion-like behavior of ceramic materials can then be compared with the chemical dissolution of the oxides into ions or complex ions of respective metallic oxide substrates. An example of this is the solubility of aluminum oxide as alumina or titanium oxide as titania. This statement is generally valid; however, most metallic oxides and nonmetallic substrates have amorphous hydroxide–inclusive structures, but bulk ceramics are mostly crystalline. The corrosion resistance of synthetic polymers, on the other hand, depends not only on their composition and structural form but also on the degree of polymerization. Unlike metallic and ceramic materials, synthetic polymers are not only dissolved but also penetrated by water and substances from biological environments. The resulting degree of alteration depends on the material property conditions for the manufactured component.
Toxicity and Considerations
Toxicity is related to primary biodegradation products (simple and complex cations and anions), particularly those of higher atomic weight metals. Factors to be considered include (1) the amount dissolved by biodegradation per time unit, (2) the amount of material removed by metabolic activity in the same time unit, and (3) the quantities of solid particles and ions deposited in the tissue and any associated transfers to the systemic system. For example, the quantity of elements released from metals during corrosion time (e.g., grams per day) can be calculated by using the following formula15:
< ?xml:namespace prefix = "mml" />
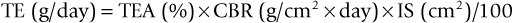
where TE = toxic element, TEA = toxic elements in alloy, CBR = corrosion biodegradation, and IS = implant surface.
It is of little importance for the formula whether or not the metallic substrate is exposed because the passive layer is dissolved. The critical issue is that the surface represents the “finished” form of the implant. The formula is also valid for ceramic materials and for substances transferred from synthetic polymers. Therefore, it appears that the toxicity is related to the content of the materials’ toxic elements and that they may have a modifying effect on corrosion rate.15
The transformation of harmful primary products is dependent on their level of solubility and transfer. It is known that whereas chromium and titanium ions react locally at low concentrations, cobalt, molybdenum, or nickel can remain dissolved at higher relative concentrations and thus may be transported and circulated in body fluids. Several studies have documented the relative toxicity of titanium and its alloys and are addressed within the section on titanium.
Lemons32 reported on the formation of electrochemical couples as a result of oral implant and restorative procedures and stressed the importance of selecting compatible metals to be placed in direct contact with one another in the oral cavity to avoid the formation of adverse electrochemical couples. The electrochemical behavior of implanted materials has been instrumental in assessing their biocompatibility.42 Zitter and Plenk43 have shown that anodic oxidation and cathodic reduction take place in different spaces but must always balance each other through charge transfer. This has been shown to impair both cell growth and transmission of stimuli from one cell to another. Therefore, an anodic corrosion site can be influenced by ion transfer but also by other possibly detrimental oxidation phenomena. Charge transfer appears to be a significant factor specific to the biocompatibility of metallic biomaterials. Passive layers along the surfaces of titanium, niobium, zirconium, and tantalum increase resistance to charge transfer processes by isolating the substrate from the electrolyte in addition to providing a higher resistance to ion transfers. On the other hand, metals based on iron, nickel, or cobalt are not as resistant to transfers through the oxidelike passive surface zones.
Metals and Alloys
To date, most of the dental implant systems available within the United States are constructed from metals or alloys. These materials are reviewed in this chapter by separating the metals and alloys according to their elemental compositions because a growing proportion have modified surface characteristics that are addressed in the second section of this chapter.
Several organizations have provided guidelines for the standardization of implant materials.44 ASTM Committee F4 (ASTM F4) and ISO (ISOTC 106, ISOTR 10541) have provided the basis for such standards.19,20 To date, a multinational survey by ISO indicated that titanium and its alloy are mainly used. The most widely used nonmetallic implants are oxidic, carbonitic, or graphitic oxidelike materials.45
The major groups of implantable materials for dentistry are titanium and alloys, cobalt chromium alloys, austenitic Fe-Cr-Ni-Mo steels, tantalum, niobium and zirconium alloys, precious metals, ceramics, and polymeric materials.
Titanium and Titanium–6 Aluminum–4 Vanadium (Ti-6Al-4V)
This reactive group of metals and alloys (with primary elements from reactive group metallic substances) form tenacious oxides in air or oxygenated solutions. Titanium oxidizes (passivates) on contact with room temperature air and normal tissue fluids. This reactivity is favorable for dental implant devices. In the absence of interfacial motion or adverse environmental conditions, this passivated (oxidized) surface condition minimizes biocorrosion phenomena. In situations in which the implant would be placed within a closely fitting receptor site in bone, areas scratched or abraded during placement would repassivate in vivo. This characteristic is one important property consideration related to the use of titanium for dental implants.37,46–48 Some reports show that the oxide layer tends to increase in thickness under corrosion testing48 and that breakdown of this layer is unlikely in aerated solutions.49
Bothe et al.50 studied the reaction of rabbit bone to 54 different implanted metals and alloys and showed that titanium allowed bone growth directly adjacent to the oxide surfaces. Leventhal51 further studied the application of titanium for implantation. Beder et al.,52 Gross et al.,53 Clarke et al.,54 and Brettle55 were able to expand indications of these materials. In all cases, titanium was selected as the material of choice because of its inert and biocompatible nature paired with excellent resistance to corrosion.1,56–60
Specific studies in the literature addressed the corrosion of titanium implants and are reported in the surface characteristics section. Unfortunately, most are for in vitro and unloaded conditions, and few identify precisely the type of titanium and titanium surface studied.
The general engineering properties of the metals and alloys used for dental implants are summarized in Table 4-1. Titanium shows a relatively low modulus of elasticity and tensile strength compared with most other alloys. The strength values for the wrought soft and ductile metallurgic condition (normal root forms and plate form implants) are approximately 1.5 times greater than the strength of compact bone. In most designs in which the bulk dimensions and shapes are simple, the strength of this magnitude is adequate. Because fatigue strengths are normally 50% weaker or less than the corresponding tensile strengths, implant design criteria are decidedly important. The creation of sharp corners or thin sections must be avoided for regions loaded under tension or shear conditions. The modulus of elasticity of titanium is five times greater than that of compact bone, and this property places emphasis on the importance of design in the proper distribution of mechanical stress transfer. In this regard, surface areas that are loaded in compression have been maximized for some of the newer implant designs. Four grades of unalloyed titanium and titanium alloy are the most popular. Their ultimate strength and endurance limit vary as a function of their composition.
TABLE 4-1
Engineering Properties of Metals and Alloys Used for Surgical Implants*
| Material | Nominal Surface Analysis (w/o) | Modulus of Elasticity, GN/m2 (psi µ 106) | Ultimate Tensile Strength, MN/m2 (ksi) | Elongation to Fracture (%) | |
| Titanium oxide | 99+Ti | 97 (14) | 240–550 (25–70) | 15 | Ti |
| Titanium oxide aluminum–vanadium | 90Ti-6Al-4V | 117 (17) | 869–896 | >12 | Ti |
| Cobalt–oxide chromium–molybdenum (casting) | 66Co-27Cr-7Mo | 235 (34) | 655 (95) | >8 | Cr |
| Stainless oxide steel (316L) | 70Fe-18Cr-12Ni | 193 (28) | 480–1000 | >30 | Cr |
| Zirconium oxide | 97 (14) | 552 (80) | 20 | Zr | |
| Tantalum oxide | — | 690 (100) | 11 | Ta | |
| Gold | 99+Au | 97 (14) | 207–310 (30–45) | >30 | Au |
| Platinum | 99+Pt | 166 (24) | 131 (19) | 40 | Pt |
* Minimum values from the American Society for Testing and Materials Committee F4 documents are provided. Selected products provide a range of properties.
GN/m2, Giganewtons per meter squared; ksi, thousand pounds per inch squared; MN/m2, meganewtons per meter squared; psi, pounds per inch squared; w/o, weight percent.
The alloy of titanium most often used is titanium–aluminum–vanadium. The wrought alloy condition is approximately six times stronger than compact bone and thereby affords more opportunities for designs with thinner sections (e.g., plateaus, thin interconnecting regions, implant-to-abutment connection screw housing, irregular scaffolds, porosities). The modulus of elasticity of the alloy is slightly greater than that of titanium, being about 5.6 times that of compact bone. The alloy and the primary element (i.e., titanium) both have titanium oxide (passivated) surfaces. Information has been developed on the oxide thickness, purity, and stability as related to implant biocompatibilities.9,14,19 In general, titanium and alloys of titanium have demonstrated interfaces described as osseointegrated for implants in humans. In addition, surface conditions in which the oxide thickness has varied from hundreds of angstroms of amorphous oxide surface films to 100% titania (titanium dioxide [TiO2] rutile form ceramic) have demonstrated osseointegration.
The possible influences of aluminum and vanadium biodegradation products on local and systemic tissue responses have been reviewed from the perspectives of basic science and clinical applications.61 Extensive literature has been published on the corrosion rate of titanium within local tissue fluids62–64 and the periimplant accumulation of “black particles.”65 A few adverse effects have been reported.66 Increased titanium concentrations were found in both periimplant tissues and parenchymal organs,67,68 mainly the lung and much lesser concentrations in the liver, kidney, and spleen.25,66–70 However, alloy compositions were not well defined or controlled. Corrosion and mechanical wear have been suggested as possible causes.48,67,68 Authors who still caution about the applicability of these results to the presently available titanium alloys have developed other alloys using iron, molybdenum, and other elements as primary alloying agents.17 More recently, several new titanium alloys of higher strength have been introduced.33,71
Although many basic science questions remain, clinical applications of these alloys in dental and orthopedic surgical systems have been very positive, especially in light of improved strength, and the titanium alloys have not demonstrated significant numbers of identifiable negative sequelae.19
Electrochemical studies support the selection of conditions in which elemental concentrations would be relatively low in magnitude.11 Electrochemically, titanium and titanium alloy are slightly different in regard to electromotive and galvanic potentials compared with other electrically conductive dental materials. Results of these electrochemical potentials and how they relate to in vivo responses have been published previously.9,42,63 In general, titanium- and cobalt-based systems are electrochemically similar; however, comparative elements imitating the conditions in an aeration cell revealed that the current flow in titanium and titanium alloys is several orders of magnitude lower than that in Fe-Cr-Ni-Mo steels or Co-Cr alloys.15
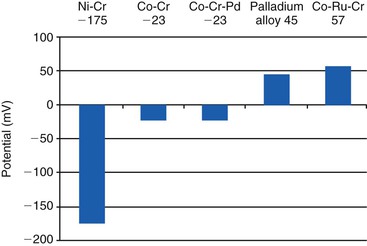
Recent reports have challenged traditional thinking in some ways regarding the use of cobalt-based alloys as superstructures for implant prosthetics. As the price of noble metals has increased, clinicians are exploring alternatives for prosthetic constructs. This is particularly true in the case of implant dentistry, in which metal substructures can be of considerable size, with a comparable cost. Dissimilar metals, when attached electrochemically, may experience ion flow, and the deleterious clinical consequences of this are noted.72,73 Because of the unique and tenacious oxide layer formed by titanium, ion flow is limited. If titanium is coupled with a gold superstructure, for example, the titanium oxide formed on the surface of the titanium prevents clinically significant ion exchange, leading to clinically acceptable intraoral couples. In couples in which the titanium is the more noble metal, the less noble metal continues to corrode, especially in crevices. In other words, the more noble metal will corrode the less noble metal (Table 4-2). The amount of corrosion and current flow depends on the particular host environment; the assessment is further complicated by varied in vitro techniques used.74 At least one in vitro study supports the use of a cobalt-based alloy coupled with titanium.75 The use of cobalt-based superstructures is further supported by a wide variety of clinical experiences and commercially available superstructures made from cobalt–chromium alloys. As one example, many large commercial laboratories mill implant superstructures out of cobalt-based alloys. Other clinicians have published case reports using cobalt-based implant superstructures.76,77
TABLE 4-2
Electrochemical Reactivity
| A | Gold alloys |
| B | Palladium alloys |
| C | Titanium alloys |
| D | Cobalt alloys |
| E | Nickel alloys |
At least one manufacturer (NobleBond; Argen) has responded to the concerns above by producing a cobalt-based alloy with large amounts of ruthenium. Ruthenium (Ru) is a noble metal in the platinum family, with excellent corrosion resistance, but is considerably cheaper than gold and platinum. This alloy contains 40% cobalt, 25% ruthenium, and 24% chromium. Although the clinical performance of this alloy remains to be seen, advances such as these may eventually permit clinicians to use alternative alloys such as palladium- and ruthenium-based metals for clinical restoration of implants (Figure 4-2). The use of palladium-based alloys is also supported by clinical use and in vitro analysis.78
To eliminate the presence of dissimilar metals, some clinicians have chosen to fabricate implant superstructures using milling techniques. With this technology, an implant bar superstructure, for example, can be milled from a single billet of titanium (Figure 4-3). Advances in optical imaging (for impressions, either in the laboratory or intraorally), as well as milling technology, have made this approach possible. Because the superstructure is milled from the same material as the implants themselves, dissimilar metals are not present, and current does not flow. Alternatively, the superstructure can be milled from all ceramic (Zirconzahn; Figure 4-4). This approach can use machined metal connectors that are luted into the structure (Figure 4-5), or the implant–abutment interface may be milled into the prosthesis as well.
Mechanically, titanium is much more ductile (bendable) than titanium alloy. This feature has been a very favorable aspect related to the use of titanium for endosteal plate form devices. The need for adjustment or bending to provide parallel abutments for prosthetic treatments has caused manufacturers to optimize microstructures and residual strain conditions. Coining, stamping, or forging followed by controlled annealing heat treatments are routinely used during metallurgic processing. However, if an implant abutment is bent at the time of implantation, then the metal is strained locally at the neck region (bent), and the local strain is both cumulative and dependent on the total amount of deformation introduced during the procedure. This is one reason, other than prior loading fatigue cycling, why reuse of implants is not recommended. In addition, mechanical processes can sometimes significantly alter or contaminate implant surfaces. Any residues of surface changes must be removed before implantation to ensure mechanically and chemically clean conditions.
The emerging techniques to cast titanium and titanium alloys remain limited for dental implant application because of high melting points of the elements and propensity for absorption of oxygen, nitrogen, and hydrogen, which may cause metallic embrittlement. A high vacuum or ultrapure protective gas atmosphere allows the production of castings in titanium and its alloys at different purity levels,79,80 although microstructures and porosity are relatively unfavorable related to fatigue and fracture strengths.9,32 Typical strengths of cast commercially pure (CP) titanium grade 2 and Ti-6Al-4V after heat treatment and annealing can be in the range of those of wrought titanium alloys used for dental implants.81
Cobalt-Chromium-Molybdenum–Based Alloy
The cobalt-based alloys are most often used in an as-cast or cast-and-annealed metallurgic condition. This permits the fabrication of implants as custom designs such as subperiosteal frames. The elemental composition of this alloy includes cobalt, chromium, and molybdenum as the major elements. Cobalt provides the continuous phase for basic properties; secondary phases based on cobalt, chromium, molybdenum, nickel, and carbon provide strength (four times that of compact bone) and surface abrasion resistance (see Table 4-1); chromium provides corrosion resistance through the oxide surface; and molybdenum provides strength and bulk corrosion resistance. All of these elements are critical, as is their concentration, which emphasizes the importance of controlled casting and fabrication technologies. Also included in this alloy are minor concentrations of nickel, manganese, and carbon. Nickel has been identified in biocorrosion products, and carbon must be precisely controlled to maintain mechanical properties such as ductility. Surgical alloys of cobalt are not the same as those used for partial dentures, and substitutions should be avoided.
In general, the as-cast cobalt alloys are the least ductile of the alloy systems used for dental surgical implants, and bending of finished implants should be avoided. Because many of these alloy devices have been fabricated by dental laboratories, all aspects of quality control and analysis for surgical implants must be followed during alloy selection, casting, and finishing. Critical considerations include the chemical analysis, mechanical properties, and surface finish as specified by the ASTM F4 on surgical implants and the ADA.19,21 When properly fabricated, implants from this alloy group have shown to exhibit excellent biocompatibility profiles.
Iron-Chromium-Nickel–Based Alloys
The surgical stainless steel alloys (e.g., 316 low carbon [316L]) have a long history of use for orthopedic and dental implant devices. This alloy, as with titanium systems, is used most often in a wrought and heat-treated metallurgic condition, which results in a high-strength and high-ductility alloy. The ramus blade, ramus frame, stabilizer pins (old), and some mucosal insert systems have been made from the iron-based alloy.
The ASTM F4 specification for surface passivation was first written and applied to the stainless steel alloys.19 In part, this was done to maximize corrosion–biocorrosion resistance. Of the implant alloys, this alloy is most subject to crevice and pitting biocorrosion, and care must be taken to use and retain the passivated (oxide) surface condition. Because this alloy contains nickel as a major element, use in patients allergic or hypersensitive to nickel should be avoided. In addition, if a stainless steel implant is modified before surgery, then recommended procedures call for repassivation to obtain an oxidized (passivated) surface condition to minimize in vivo biodegradation.
The iron-based alloys have galvanic potentials and corrosion characteristics that could result in concerns about galvanic coupling and biocorrosion if interconnected with titanium, cobalt, zirconium, or carbon implant biomaterials.82–84 In some clinical conditions, more than one alloy may be present within the same dental arch of a patient. For example, if a bridge of a noble or a base-metal alloy touches the abutment heads of a stainless steel and titanium implant simultaneously, then an electrical circuit would be formed through the tissues. If used independently, where the alloys are not in contact or not electrically interconnected, then the galvanic couple would not exist, and each device could function independently. As with the other metal and alloy systems discussed, the iron-based alloys have a long history of clinical applications. Long-term device retrievals have demonstrated that, when used properly, the alloy can function without significant in vivo breakdown. Clearly, the mechanical properties and cost characteristics of this alloy offer advantages with respect to clinical applications.
Other Metals and Alloys
Many other metals and alloys have been used for dental implant device fabrication. Early spirals and cages included tantalum, platinum, iridium, gold, palladium, and alloys of these metals. More recently, devices made from zirconium, hafnium, and tungsten have been evaluated.15,85,86 Some significant advantages of these reactive group metals and their alloys have been reported, although large numbers of such devices have not been fabricated in the United States.
Gold, platinum, and palladium are metals of relatively low strength, which places limits on implant design. In addition, cost-per-unit weight and the weight-per-unit volume (density) of the device along the upper arch have been suggested as possible limitations for gold and platinum. These metals, especially gold because of nobility and availability, continue to be used as surgical implant materials. For example, the Bosker endosteal staple design represents use of this alloy system.87
Ceramics and Carbon
Ceramics are inorganic, nonmetallic, nonpolymeric materials manufactured by compacting and sintering at elevated temperatures. They can be divided into metallic oxides and other compounds. Oxide ceramics were introduced for surgical implant devices because of their inertness to biodegradation, high strength, physical characteristics such as color and minimal thermal and electrical conductivity, and a wide range of material-specific elastic properties.88,89 In many cases, however, the low ductility or inherent brittleness has resulted in limitations. Ceramics have been used in bulk forms and more recently as coatings on metals and alloys.
Aluminum, Titanium, and Zirconium Oxides
High-strength ceramics from aluminum, titanium, and zirconium oxides have been used for root form, endosteal plate form, and pin type of dental implants.90 The overall characteristics of these ceramics are summarized in Table 4-3. The compressive, tensile, and bending strengths exceed the strength of compact bone by three to five times. These properties, combined with high moduli of elasticity, and especially with fatigue and fracture strengths, have resulted in specialized design requirements for these classes of biomaterials.19,91 For example, the fabrication of a subperiosteal device from a high ceramic should not be done because of the custom nature of these devices, the lower fracture resistance, and the relative cost for manufacturing. The aluminum, titanium, and zirconium oxide ceramics have a clear, white, cream, or light-gray color, which is beneficial for applications such as anterior root form devices. Minimal thermal and electrical conductivity, minimal biodegradation, and minimal reactions with bone, soft tissue, and the oral environment are also recognized as beneficial compared with other types of synthetic biomaterials. In early studies of dental and orthopedic devices in laboratory animals and humans, ceramics have exhibited direct interfaces with bone, similar to an osseointegrated condition with titanium. In addition, characterization of gingival attachment zones along sapphire root form devices in laboratory animal models has demonstrated regions of localized bonding.9,92–96
TABLE 4-3
Engineering Properties of Some Inert Ceramics Used as Biomaterials*
| Material | Modulus of Elasticity, GN/m2 (psi µ 106) | Ultimate Bending Strength, MPa (ksi) | Surface |
| Aluminum oxide (43–80) | Polycrystalline Al2O3 | 372 (54) | 300–550 |
| Single crystal (sapphire) Al2O3 | 392 (56) | 640 (93) | |
| Zirconium oxide zirconia (PSZ) (72–94) | ZrO2 | 195–210 (28–30) | 500–650 |
| Titanium oxide (titania) | 280 (41) | 69–103 (10–15) | TiO2 |
* These high ceramics have 0% permanent elongation at fracture.
GN/m2, Giganewtons per meter squared; ksi, thousand pounds per inch squared; MPa, megapascals; psi, pounds per inch squared.
Although the ceramics are chemically inert, care must be taken in the handling and placement of these biomaterials. Exposure to steam sterilization results in a measurable decrease in strength for some ceramics; scratches or notches may introduce fracture initiation sites; chemical solutions may leave residues; and the hard and sometimes rough surfaces may readily abrade other materials, thereby leaving a residue on contact. Dry-heat sterilization within a clean and dry atmosphere is recommended for most ceramics.
One series of root form and plate form devices used during the 1970s resulted in intraoral fractures after several years of function.97 The fractures were initiated by fatigue cycling where biomechanical stresses were along regions of localized bending and tensile loading. Although initial testing showed adequate mechanical strengths for these polycrystalline alumina materials,98 the long-term clinical results clearly demonstrated a functional design-related and material-related limitation. This illustrates the need for controlled clinical investigation to relate basic properties to in vivo performance. The established chemical biocompatibilities, improved strength and toughness capabilities of sapphire and zirconia, and the basic property characteristics of high ceramics continue to make them excellent candidates for dental implants.
Bioactive and Biodegradable Ceramics Based on Calcium Phosphates
Bone Augmentation and Replacement
The calcium phosphate (CaPO4) materials (i.e., calcium phosphate ceramics [CPCs]) used in dental reconstructive surgery include a wide range of implant types and thereby a wide range of clinical applications. Early investigations emphasized solid and porous particulates with nominal compositions that were relatively similar to the mineral phase of bone (Ca5[PO4]3OH). Microstructural and chemical properties of these particulates were controlled to provide forms that would remain intact for structural purposes after implantation. The laboratory and clinical results for these particulates were most promising and led to expansions for implant applications, including larger implant shapes (e.g., rods, cones, blocks, H-bars) for structural support under relatively high-magnitude loading conditions.99,100 In addition, the particulate size range for bone replacements was expanded to both smaller and larger sizes for combined applications with organic compounds. Mixtures of particulates with collagen, and subsequently with drugs and active organic compounds such as bone morphogenetic protein, increased the range of possible applications. Over the past 20 years, these types of products and their uses have continued to expand significantly.100–103
Endosteal and Subperiosteal Implants
The first series of structural forms for dental implants included rods and cones for filling tooth root extraction sites (ridge retainers)104 and, in some cases, load-bearing endosteal implants.105 Limitations in mechanical property characteristics soon resulted in internal reinforcement of the CPC implants through mechanical (central metallic rods) or physicochemical (coating over another substrate) techniques.106,107
The numbers of coatings of metallic surfaces using flame or plasma spraying (or other techniques) increased rapidly for the CPCs.100 The coatings have been applied to a wide range of endosteal and subperiosteal dental implant designs, with an overall intent of improving implant surface biocompatibility profiles and implant longevities (and are addressed later in this chapter).108–110
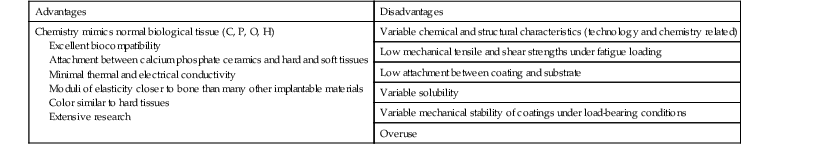
Advantages and Disadvantages
Box 4-1 summarizes the advantages and disadvantages of CPCs. The recognized advantages associated with the CPC biomaterials are as follows111:
2. Excellent biocompatibility profiles within a variety of tissues, when used as intended
3. Opportunities to provide attachments between selected CPC and hard and soft tissues
6. Color similar to bone, dentin, and enamel
7. An evolving and extensive base of information related to science, technology, and application
Some of the possible disadvantages associated with these types of biomaterials are as follows:
1. Variations in chemical and structural characteristics for some currently available implant products
2. Relatively low mechanical tensile and shear strengths under condition of fatigue loading
3. Relatively low attachment strengths for some coating-to-substrate interfaces
6. Expansion of applications that sometimes exceed the evolving scientific information on properties
Critical to applications are the basic properties of these substances. Table 4-4 provides a summary of some properties of bioactive and biodegradable ceramics. In general, these classes of bioceramics have lower strengths, hardnesses, and moduli of elasticity than the more chemically inert forms previously discussed. Fatigue strengths, especially for porous materials, have imposed limitations with regard to some dental implant designs. In certain instances, these characteristics have been used to provide improved implant conditions (e.g., biodegradation of particulates).
TABLE 4-4
Properties of Bioactive and Biodegradable Ceramics*
| Material | Modulus of Elasticity, GPa (psi µ 106) | Ultimate Bending Strength, MPa (ksi) | Surface |
| Hydroxyapatite | 40–120 (6–17) | 40–300 (6–43) | Ca10(PO4)6(OH)2 |
| Tricalcium phosphate | 30–120 (4–17) | 15–120 (2–17) | Ca3(PO4)2 |
| Bioglass or Ceravital | 40–140 (6–20) | 20–350 (3–51) | CaPO4 |
| AW ceramic | 124 (18) | 213 (31) | CaPO4 + F |
| Carbon | 25–40 (4–6) | 150–250 (22–36) | C |
| Carbon–silicon (LTI) | 25–40 (4–6) | 200–700 (29–101) | CSi |
* These ceramics and carbons have 0% permanent elongation at fracture.
GPa, Gigapascals; psi, pounds per inch squared; ksi, thousand pounds per inch squared; LTI, low-temperature isotropic; MPa, megapascals.
Calcium aluminates, sodium–lithium invert glasses with CaPO4 additions (Bioglass or Ceravital), and glass ceramics (AW glass-ceramic) also provide a wide range of properties and have found extended applications.103,107
Bioactive Ceramic Properties
Physical properties are specific to the surface area or form of the product (block, particle), porosity (dense, macroporous, microporous), and crystallinity (crystalline or amorphous). Chemical properties are related to the calcium–phosphate ratio, composition, elemental impurities (e.g., carbonate), ionic substitution in atomic structure, and the pH of the surrounding region. These properties plus the biomechanical environment all play a role in the rate of resorption and the clinical application limits of the materials.
The atomic relationships of the basic elements, stoichiometric ratios, and the normal chemical names for several characterized CPCs are provided in Table 4-5. The general family of apatites has the following formula:
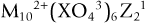
TABLE 4-5
Names, Formulae, and Atomic Ratios for Some Calcium Phosphate Materials
| Mineral or General Name | Formula | Ca : P Ratio | Applications |
| Monetite (DVP) substitute particulate | CaHPO4 | 1 | Nonceramic bone |
| Brushite (DCPD) biomaterials | CaHPO4 2H2O | 1 | Phase of some CaPO4 |
| Octacalcium phosphate (OCP) | Ca8(HPO4)2(PO4) 5H2O | 11.33 | Phase of some CaPO4 biomaterials |
| Whitlockite (WH) biomaterials | Ca10(HPO4)(PO4)6 | 1.43 | Phase of some CaPO4 biomaterials |
| Beta-tricalcium phosphate (b-TCP) | Ca3(PO4)2 | 1.48 | Biodegradable CaPO4 ceramic for bone substitute and coatings; also a phase of some CaPO4 biomaterials |
| Defective hydroxyapatite (DOHA) biomaterials | Ca9(HPO4)(PO4)5(OH) | 1.5 | Component of some CaPO4 biomaterials |
| Hydroxyapatite (HA) | Ca10(PO4)6(OH)2 | 1.67 | Major mineral phase of bone; when fired as a ceramic, named HA |
Very often, apatite atomic ratios are nonstoichiometric; that is, 1 mol of apatite may contain fewer than 10 mol of metallic ions (M2+) and fewer than 2 mol of anions Z−1.112 The number of XO retains a number of 6. Multiple metals and anions can be substituted within this formulation. Most important, the relative physical, mechanical, and chemical properties of each final CaPO4 material, including each of the apatites, are different from one another.96,102 In addition, the microstructure of any final product (solid structural form or coating) is equally important to the basic properties of the substance alone. The crystalline monolithic hydroxyapatite (HA) (fired ceramic Ca10[PO4]6[OH]2) of high density and purity (50 maximum ppm impurities) has provided one standard for comparison related to implant applications. The ratio of calcium to phosphorus of Ca10(PO4)6(OH)2 is 1.67, and the ceramic can be fully crystalline. Considerable differences exist between the synthetic HA ceramics (HAs) that are produced by elevated temperature processing and biological apatites (HAs).112 Biological apatites contain trace amounts of (CO3)2, sodium, magnesium, fluorine, and chlorine ions. These exist in varying ratios and distributions and, of course, are only one phase of calcified tissues.
The crystalline tricalcium phosphate (bCa3[PO4]2) (b-TCP) ceramic has also provided a high-purity (<50 ppm maximum impurities) biomaterial for comparison with other products. National standard specifications related to the basic properties and characteristics of both HA and TCP have been published.19 These two compositions have been used most extensively as particulates for bone augmentation and replacement, carriers for organic products, and coatings for endosteal and subperiosteal implants.
One of the more important aspects of the CPCs relates to the possible reactions with water. For example, hydration can convert other compositions to HA; also, phase transitions among the various structural forms can exist with any exposure to water. This has caused some confusion in the literature in that some CPCs have been steam autoclaved for sterilization purposes before surgical implantation. Steam or water autoclaving can significantly change the basic structure and properties of CPCs (or any bioactive surface) and thereby provide an unknown biomaterial condition at the time of implantation. This is to be avoided through the use of presterilized or clean, dry heat or gamma-sterilized conditions.
Forms, Microstructures, and Mechanical Properties
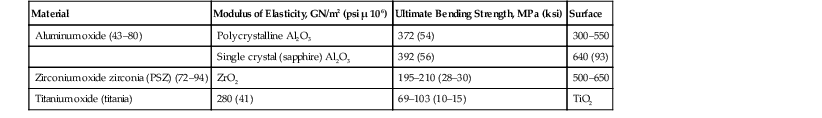
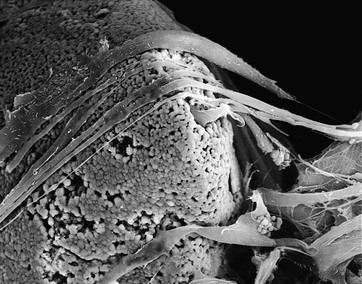
Particulate HA, provided in a nonporous (<5% porosity) form as angular or spherically shaped particles, is an example of a crystalline, high-purity HA biomaterial113 (Figure 4-6, A). These particles can have relatively high compressive strengths (up to 500 MPa), with tensile strengths in the range of 50 to 70 MPa. Usually, dense polycrystalline ceramics consisting of small crystallites exhibit the highest mechanical strength apart from monocrystalline ceramics free of defects (e.g., single-crystal sapphire implants). Ceramics are brittle materials and exhibit high compressive strengths compared with tensile strengths. However, less resistance to tensile and shear stresses limit their application as dental implants because of mechanical constraints of implant form and volume. Nonresorbable, “bioinert” ceramics exhibiting satisfactory load-bearing capability are limited to dense monocrystalline and polycrystalline aluminum, zirconium, and titanium oxide ceramics. These same mechanical characteristics exist for the solid portions of several porous HA particulates and blocks. The macroporous (>50 mm) or microporous (<50 mm) particulates have an increased surface area per unit volume. This provides more surface area for solution- and cell-mediated resorption under static conditions and a significant reduction in compressive and tensile strengths (Figure 4-6, B and C; Figure 4-7). The porous materials also provide additional regions for tissue ingrowth and integration (mechanical stabilization) and thereby a minimization of interfacial motion and dynamic (wear-associated) interfacial breakdown. The strength characteristics after tissue ingrowth would then become a combination of the ceramic and the investing tissues.114
A number of the CPCs are phase mixtures of HA and TCP, but some compounds are composites or mechanical mixtures with other materials100 (see Table 4-5). These classes of bioactive ceramics, including glasses, glass-ceramics, mixtures of ceramics, combinations of metals and ceramics, and polymers and ceramics, exhibit a wide range of properties. In general, these biomaterials have shown acceptable biocompatibility profiles from laboratory and clinical investigations. Bulk-form implant designs made from CPCs, which were contraindicated for some implant designs because of poor mechanical performance, have found a wide range of indications as coatings of stronger implant materials.
The coatings of CPCs onto metallic (cobalt- and titanium-based) biomaterials have become a routine application for dental implants. For the most part these coatings are applied by plasma spraying, have average thickness between 50 and 70 mm, are mixtures of crystalline and amorphous phases, and have variable microstructures (phases and porosities) compared with the solid portions of the particulate forms of HA and TCP biomaterials.100,115 At this time, coating characteristics are relatively consistent, and the quality control and stricter quality assurance programs from the manufacturers have greatly improved the consistency of coated implant systems. (A more detailed discussion of surface treatment options is presented in the next section.)
Concerns continue to exist about the fatigue strengths of the CaPO4 coatings and coating–substrate interfaces under tensile and shear loading conditions. There have been some reports of coating loss as a result of mechanical fracture, although the numbers reported remain small.96 This has caused some clinicians and manufacturers to introduce designs in which the coatings are applied to shapes (geometric designs) that minimize implant interface shear or tensile loading conditions (e.g., porosities, screws, spirals, plateaus, vents). From theoretical considerations, the coating of mechanically protected areas seems most desirable.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses