Chapter 36 Keys to Bone Grafting and Bone Grafting Materials
To satisfy the ideal goals of implant dentistry, the hard and soft tissues need to present ideal volumes and quality. The alveolar process is affected so often after tooth loss that augmentation is usually indicated to achieve optimum results, especially in the esthetic zones. Augmentation is also required relative to functional conditions of the implant treatment plan, because a reduction of stress at the crestal bone region and a greater resistance to screw loosening and fatigue fracture occurs with larger-diameter implants. Therefore an improved understanding of biomechanical requirements for long-term prosthesis survival and the increasing use of implants in esthetic restorations often require ridge reconstruction before implant placement for complete or partially edentulous patients. This is especially true in the surgical placement of maxillary anterior implants, which is usually critical for ideal esthetics, phonetics, and function. As a result, treatment methods to improve the recipient bone dimensions to optimize success should be considered, especially in the premaxilla.
ALVEOLAR RESORPTION
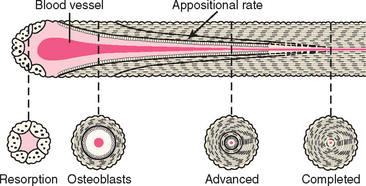
In the anterior maxilla, the alveolar bone is rapidly recontoured after the loss of the natural teeth, even in the presence of an intact alveolus after extraction. There is a 25% decrease in volume during the first year and a 40% to 60% decrease in width within the first 3 years after tooth loss.1,2 This resorption is primarily at the expense of the facial dimension, as the lingual contour undergoes resorption only when more advanced atrophy occurs. As a result, an 8-mm-wide anterior ridge may remodel to less than 3 mm within 5 years after extraction.3,4 In the posterior regions, the rate of initial bone loss is often greater than in the anterior regions. However, because the initial posterior ridge dimension is twice the width, even a 50% bone loss often leaves adequate volume to place 4-mm-diameter implants. As a consequence of these dimensional changes, the remodeled labial cortex is more medial than its original position.
SURGICAL KEYS TO BONE GRAFTING
Consistent bone grafting results for volume have been difficult to achieve, often because similar techniques are used, regardless of the patient’s existing conditions, the volume of bone, and the region of the augmentation. Specific elements (keys) need to be present for successful bone grafting.5 The doctor should evaluate the existing condition and alter the grafting technique and materials in function of each treatment performed. In other words, bone grafting is very much like opening a combination safe that requires at least seven of 11 different numbers. If only five or six numbers are correct, the safe will not open. The more numbers used to open the “safe” of successful bone grafting, the more predictable the growth of sufficient bone volume for implant placement.
The keys to bone grafting are local factors that affect the prognosis of the procedure and include: absence of infection, soft tissue closure, space maintenance, graft immobilization, regional acceleratory phenomenon (RAP), host bone vascularization, growth factors, bone morphogenetic proteins (BMPs), healing time, defect size and topography, and transitional prostheses. Several of these keys are interrelated; often, one key affects another and may form a cascade toward failure or success. The surgeon should attempt to provide all these elements, but especially those factors missing in the bone augmentation site.
Surgical Asepsis/Absence of Infection
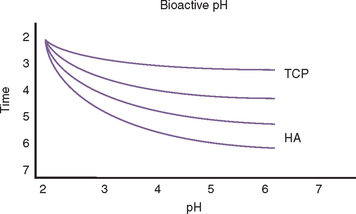
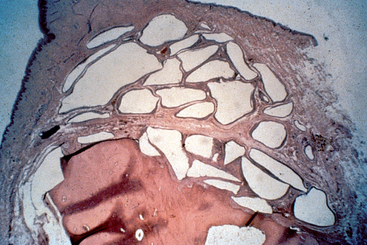
Bone and graft materials resorb at different rates under normal pH conditions, based upon porosity, size, and crystallization. However, all graft materials rapidly resorb through solution-mediated resorption in conditions of low pH (Figure 36-1). The hydroxylapatite crystal of bone (or enamel) is dissolved into calcium and phosphate components at a pH of 5.5 or less. For example, the enamel of a tooth is composed of 95% dense, crystalline hydroxylapatite.
Lactobacillus acidophilus bacteria produce a pH of 5.5, and when bacterial plaque and bacteria remain in enamel for more than 5 days, a solution-mediated resorption occurs, which is seen as a radiolucent zone on the radiograph. Infections within the bone often create a pH of less than 2. As a result, when a tooth becomes nonvital, a solution-mediated resorption occurs at the apex and a radiolucency at the apex of the tooth with an endodontic lesion may occur within a few days. Therefore bone grafting in the presence of infection, or infected bone grafts after surgery, increase the risk of insufficient volumes of bone formation and may even cause recipient bone loss (Figure 36-2). Before bone grafting, all evidence or potential causes of infection should be eliminated.
Contamination of the bone graft may occur from endogenous bacteria, lack of aseptic surgical technique, or failure of primary soft tissue closure. Graft materials that fall into the oral cavity may be contaminated by saliva and should be thoroughly irrigated before use or discarded. The lack of primary soft tissue closure or incision line opening places the graft at significant risk. Barrier membranes or fixation screws that become exposed often become contaminated by bacteria. The bacteria invade the graft site and cause local inflammation with resultant decrease in bone formation.6–10
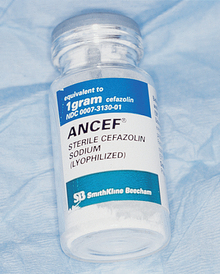
A blood supply within the graft is required for the normal distribution of an antibiotic to the site. Because no blood supply is present early on in the graft material, when bacterial contamination is a greater risk (i.e., sinus grafting), antibiotics may be added to the alloplastic material and autograft. Although tetracycline is often used in periodontal bone grafting to improve collagen formation, it chelates calcium and arrests the bone formation process.11–13 Instead, parenteral penicillin, cephalosporin, or clindamycin may be mixed into the graft material, as these antibiotics do not affect the bone regeneration process (Figure 36-3). Tablets or capsules for oral administration of antibiotics are not used in the graft site, as these often contain fillers of no benefit to the graft site.
Soft Tissue Coverage (Submucosal Space Technique) and Flap Design
Incision line opening during initial healing is the most common postoperative complication in intraoral bone grafting.14–16 As a result, the graft is contaminated or lost, vascularization is delayed or eliminated, and bone growth is impaired.8–10,17–20 The reason incision line opening is more common during bone grafting, compared with implant surgery, is that the overlying tissue must be advanced over a larger volume of bone and the tension on the incision line may pull the soft tissues apart. In addition, the soft tissues are poor in local growth factors under the reflected flaps that lie over a graft material or barrier membrane, rather than the host bone.
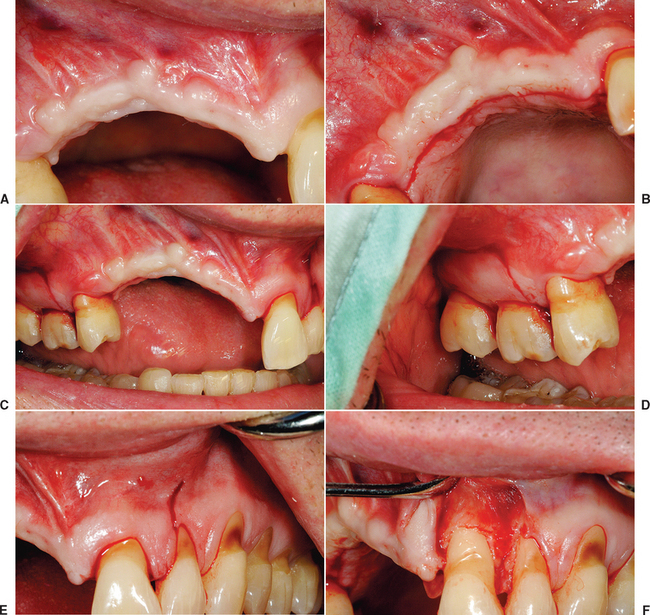
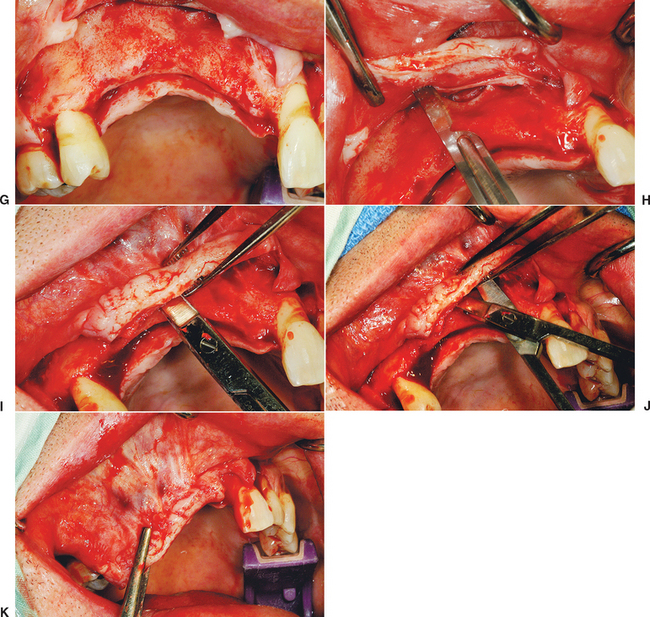
There are general guidelines to reduce the incidence of incision line opening.21 The primary incision should be in keratinized tissue whenever possible. Not only does this reduce the initial intraoral bleeding, but it severs smaller blood vessels and reduces postoperative edema, which may add tension to the incision line. The crestal incision is designed more lingual, especially in the maxilla, as this places a greater amount of keratinized tissue onto the thinner facial flap and minimizes tearing of the tissue during suturing. Vertical relief incisions are designed away from the graft site directly on the host bone and create a broad-based flap21,22 (Figure 36-4, A to C).
The blood supply to the reflected flap should be maintained whenever possible. The primary blood supply to the facial flap, which is most often the flap reflected for a bone graft, is from the unkeratinized mobile mucosa. This is especially true where muscles of facial expression or functional muscles attach to the periosteum. Therefore these vertical release incisions are made to the height of the mucogingival junction, and the facial flap is reflected only 5 mm above the height of the mucogingival junction. Both these procedures maintain more blood supply to the facial flap. In addition, incisions and reflection in the mobile alveolar mucosa increase flap retraction during initial healing, which may contribute to incision line opening and may increase risk of scar formation and delayed healing of the incision line as a consequence of reduced blood supply (see Figure 36-4, D, E).
The soft tissue flap design should also have the margins of the wound over host bone, rather than on the bone graft or barrier membrane. The host bone provides growth factors to the margins and allows the periosteum to regenerate faster to the site. The margins distal to the elevated flap should have minimal reflection. The palatal flap and the facial tissues distal to the reflected flap should not be elevated from the palatal bone (unless augmentation is required), because the blood supply to the incision line will be delayed. In addition, the unreflected flap does not retract during initial healing, which could place additional tension on the incision line. The soft tissue reflection distal to the graft site is split thickness to maintain some of the periosteum on the bone around the incision line. This improves the early vascularization to the incision line and adhesion of the margins to reduce retraction during initial healing (see Figure 36-4, F, G).
A submucosal space technique, developed by Misch in the early 1980s, is an effective method to expand tissue over larger grafts (greater than 15 × 10 mm in height and width).5 The full-thickness facial flap first is elevated off the facial bone for only 5 mm above the height of the vestibule. One incision with a scalpel, 1 to 2 mm deep, is made through the periosteum parallel to the crestal incision and 3 to 5 mm above the vestibular height of the mucoperiosteum. This shallow incision is made the full length of the facial flap and may even extend above and beyond the vertical release incisions (see Figure 36-4, H). Care is taken to make this incision above the microgingival junction; otherwise, the flap may be perforated and delay soft tissue healing. Soft tissue scissors (i.e., Metzenbaum) are used in a blunt dissection technique to create a tunnel apical to the vestibule and above the unreflected periosteum. The scissors are closed and pushed through the initial scalpel incision approximately 10 mm deep, then opened. This submucosal space is parallel to the surface mucosa (not deep toward the overlying bone) and above the unreflected periosteum. The thickness of the facial flap should be 3 to 5 mm, because the scissors are parallel to the surface (see Figure 36-4, I, J). This tunnel is expanded with the tissue scissors several millimeters above and distal to the vertical relief incisions.
Once the submucosal space is developed, the flap may now advance the distance of the “tunnel” and drape over the graft, to approximate the tissue for primary closure without tension (see Figure 36-4, K). In fact, the facial flap should be able to advance over the graft and past the lingual flap margin by more than 5 mm. Then the facial flap may be returned to the lingual flap margin and sutured. This soft tissue procedure is performed before preparing the host region and harvesting the donor site. Access to the facial tissues is easier before graft placement, and particulate graft and membranes barrier are not dislodged during the soft tissue manipulation when it is performed before bone grafting.
The submucosal space technique is very effective to achieve tension-free closure over large graft sites. However, a side effect of the procedure is the loss of vestibular depth, especially when grafting for residual ridge height. In addition, a lack of keratinized tissue also may exist on the facial region of the grafted site, because the original facial tissue is now part of the crestal region after bone augmentation. As a consequence, soft tissue grafts or vestibuloplasties may be required after bone grafting when within the esthetic zone. It is suggested that these procedures be delayed for at least 4 months to allow regeneration of the blood supply to the soft tissue and the underlying bone. When necessary, these soft tissue procedures can be associated with implant placement or implant uncovery procedures, or even prior to the bone graft. To have mature tissue for the soft tissue advancement procedures, the soft tissue graft should be done 3 months or longer before the bone graft.
Platelet-rich plasma (PRP) may be placed over the bone graft to provide an additional source of transforming growth factor beta (TGF-β) and vascular endothelial growth factor (VEGF), which promote collagen formation and blood vessel growth (Figure 36-5).
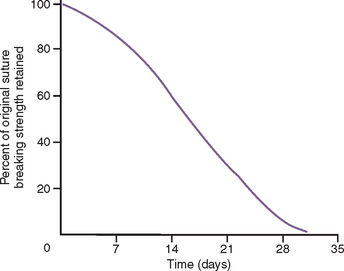
Suture material selection should be made in function of the type and size of the bone grafting.23–30 Silk has been shown to release less tension during early retraction of the flap from healing and to elicit greater inflammation and may contribute to incision line opening more often than synthetic materials.26,27 Therefore it is not recommended for bone augmentation procedures. Chromic gut causes inflammation, loses tension, and resorbs too quickly to maintain soft tissue approximation over an augmented site. Therefore it is not recommended when the tissues are advanced for a bone augmentation.29 Polyglycolic acid (PGA, Vicryl) shows the mildest tissue reaction and maintains sufficient tension over the first 2 weeks to be used for most bone graft procedures.28 In larger-size bone grafts, the soft tissue is often approximated for primary closure with nonresorbable sutures (e.g., Prolene, Gore-Tex). Resorbable sutures usually lose 50% of their tensile strength after 14 days and may be associated with a delayed incision line opening (Figure 36-6). Each penetration of the tissue by the suture causes an approximate 1-mm devital zone and the need for repair. When the sutures are too close to the margin, the devital zone may include the incision line. The nonresorbable sutures remain in place for 2 or more weeks to enhance soft tissue maturation.30
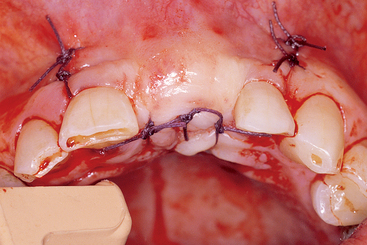
The selection of a specific suture design also follows basic principles.23,24 Interrupted sutures should be used 3 to 5 mm from each side of the tissue margin and 3 to 5 mm apart, and are appropriate in short spans of edentulous spaces (Figure 36-7). Too many sutures or too much tension impair the blood supply to the incision line and increase the risk of incision line opening. Because the tissues are passive while in place, sutures are not required to obtain the soft tissue closure. Soft tissue spans necessitating four or more interrupted sutures are best approximated with continuous nonlocking sutures. This suture design places less tension on the sutures and soft tissue and allows faster vascularization of the reflected soft tissue flaps.
Horizontal (or vertical) mattress sutures allow greater tension to be applied on the soft tissue closure without risk of tearing the soft tissue flap. It should be emphasized that they are not used to obtain primary closure when tension on the soft tissue flaps is present at surgery. The tissues should rest passively together before suturing. However, during function/parafunction movement of the tissues, the tension on the incision line may be reduced with a horizontal mattress suture. They are often used in the mandible when the floor of the mouth is in proximity to the lingual flap and the tissue is thin. They may also be used on a facial flap with a strong muscle pull on the soft tissue. In addition, horizontal mattress sutures avert the soft tissue margin and ensure primary closure without epithelium entrapment. A combination of a few horizontal mattress sutures with a continuous suture may be indicated to close large soft tissue spans.
Systemic corticosteroids may be administered before and after surgery to decrease soft tissue edema, as edema has been shown to contribute to incision line opening. Incision line opening has been associated with postoperative smoking16,31–33; therefore patients are instructed not to smoke until the incision line has healed. If a removable soft tissue-borne interim prosthesis is used, it should not contact the grafted area. When possible, a fixed transitional restoration is designed to reduce complications of incision line opening and graft micromovement during healing.
Space Maintenance
Space maintenance in the area of the bone graft site is paramount to the bone formation process. The space of the graft site refers to the anatomical size and contour of the desired augmentation, and maintenance refers to the fact the space must exist long enough for bone to fill the desired region. A barrier membrane is a sheet of material that covers the potential bone graft site and prevents the overlying soft tissue from growing into the graft site. A barrier membrane technique without graft material underneath has been suggested for ridge augmentation.18,34–38 However, collapse of the space under the membrane may impair the desired size and contour. A fixation screw, elevated above the host bone level to the height/width of the desired bone volume, may support a barrier membrane and is called a tent screw. When the space under the barrier membrane is only air or blood, the initial quality of bone is poor for many months. Tent screws, titanium-reinforced membranes, and graft material beneath the membrane have been advocated to maintain the desired space during the augmentation process.39–47 Space maintenance may be provided in a socket or sinus cavity by resorbable graft material. However, if the material resorbs too rapidly compared with the time required for bone formation, the site may fill with connective tissue rather than bone. Therefore the space or contour and size of the augmentation should remain until the bone graft has formed enough new bone to maintain the space itself.
The space for bone regeneration may also be provided by a graft material, as an autograft or alloplast “in excess.”48 The “barrier by bulk” concept by Misch applies to situations in which the graft site is overcontoured by several millimeters with a resorbable alloplast.5 As bone grows below the alloplast, the invading fibrous tissue only invades the superficial alloplast layer. When the soft tissue is reflected to insert the implants, the top layer of fibrous tissue and alloplast is removed, and the new regenerated bone underneath remains (Figure 36-8). This technique works best when larger graft volumes still allow primary soft tissue closure and in the absence of pressure of the soft tissue. The use of soft tissue-borne provisional prostheses is discouraged for all augmentation procedures because the graft size may be modified and the graft may become mobile.
Graft Immobilization/Stability/Fixation
Graft stabilization is paramount to obtain a predictable bone augmentation. This ensures initial blood clot adhesion with its associated growth factors49–56 (cytokines such as interleukin-1, interleukin-8, tumor necrosis factor, and growth factors such as plateletderived growth factor [PDGF], insulin-like growth factor, and fibroblast growth factor [FGF]).53–56 The granulation tissue that develops after blood clot stabilization is the initial mechanism for bone modeling and remodeling.53,54 If pieces of a particulate graft material or block bone grafts are mobile, they cannot develop a blood supply for new bone formation. Instead, the graft becomes encapsulated in fibrous tissue and often sequestrates. Likewise, when barrier membranes or fixation screws become loose or mobile, fibrous tissue will encapsulate them. Therefore, for barrier membranes or particulate graft materials to work most effectively, no loads should be placed on the soft tissue over the graft, which may cause movement of the graft.
Barrier membranes may be immobilized to the graft site by bone tacks. As a result, when the soft tissue over the barrier membrane moves, the barrier membrane remains fixated. A tent screw placed under the barrier membrane can prevent movement to the graft site when the overlaying soft tissue is loaded. A block bone graft fixation with bone screws maintains a rigid interface more effectively than particulate grafts (Figure 36-9). A fixed transitional prosthesis, which protects the graft site, is the better option to limit graft movement during the augmentation process, especially with a “barrier by bulk” technique of particulate graft materials. When possible, soft tissue-bone removable restorations should have rest seats and clasps to prevent loading the soft tissue (Figure 36-10).
Regional Acceleratory Phenomenon
The RAP is the local response to a noxious stimulus and describes a process by which tissue forms faster than the normal regional regeneration process.57–61 By enhancing the various healing stages, this phenomenon makes the healing process occur two to 10 times faster than normal physiologic healing. The RAP begins within a few days of injury, typically peaks at 1 to 2 months, usually lasts 4 months in bone, and may take 6 to more than 24 months to subside.55–57 The duration and intensity of the RAP are directly proportional to the type and amount of stimulus and the site where it was produced. For bone injuries, the degree of remodeling activity varies depending on the extent of bone injury, the quantity of soft tissue involved in the injury, and the configuration of the bone fracture or trauma.
Noxious stimuli of sufficient magnitude, such as fractures, mechanical abuses, and noninfectious inflammatory injuries (including dental implant procedures), can evoke an RAP. Bone grafting surgery and internal fixation procedures also produce an RAP.57 In animals, an RAP has been shown to exist after mucoperiosteal surgery on mandibular bone, with a clear correlation between quantity and quality of the noxious stimuli and degree of the RAP response.62–64 When bone graft augmentation is required on both the buccal and lingual aspects, the RAP is created on both sides of the ridge. The host site during a bone graft procedure should be decorticated by drilling holes in the cortical bone. These holes provide access for trabecular bone blood vessels to the graft site, expedite revascularization, and bring growth factors to the graft site.65,66 The marrow provides differentiated cells that evolve into osteoclasts and osteoblasts. The surgical trauma increases the RAP process, which, among other factors, includes the platelets released from the damaged vessels, which release PDGF and TGF, and increase the availability of osteogenic cells in the graft site. The penetrating holes in the cortical plate also improve graft union to the host bone, which is important when implants are placed within the seam of the donor and host site region.
The host bone decortication process may use a 20:1 low-speed hand piece at 2500 rpm with a bone drill designed for fixation screws to perforate the host bone with holes 3 to 5 mm apart and should be performed under copious amounts of saline irrigation to prevent surgical heat trauma, which delays healing (Figure 36-11). Excess heat transfers several millimeters within the bone and causes thermal necrosis, which could damage bone and blood vessels needed for repair. When injury to the bone is due to a pathologic process (e.g., arthrofibrosis, neuropathic soft tissue problems, rheumatoid phenomena, secondary osteoporosis, excessive heat), the RAP is either delayed or not initiated, and a complete healing process may not occur. When the RAP is inadequate, the result is a slow callus formation that is replaced by lamellar bone. This process contributes to the formation of biologically delayed unions and nonunion.
The increased rate of new formation of bone caused by the RAP does not result in a change in bone volume. In other words, the RAP in and of itself is usually restricted to bone remodeling.67 In addition, RAP is more evident in cortical bone because the normal turnover of bone cells is 2% compared with 18% for trabecular bone. Biochemical agents, such as prostaglandin E1 and bisphosphonate, also appear to facilitate the RAP.62,68
The RAP is usually accompanied by a systemic response, defined as the systemic acceleratory phenomenon (SAP), that demonstrates a metabolic response similar to the local response.69 Inadequate RAP is also associated with several systemic medical conditions, including diabetes mellitus, peripheral neuropathies, regional sensory denervation, severe radiation damage, and severe malnutrition.
Host Bone Blood Vessels
The host bone blood vessels that grow into a bone graft are of primary importance for predictable bone augmentation.70 These arteries can grow rather rapidly, compared with other tissues. Fibrous tissue may grow 1 mm each day, whereas woven bone grows at a rate of 60 μm each day. It would appear then that fibrous tissue would always win the race to fill a bony void. Yet, bone forms in an extraction socket when surrounded by walls of bone. One primary reason is that the blood vessels from the surrounding walls grow rapidly into the void and determine what type of tissue will form in the extraction site. The maxillary sinus region forms bone predictably, almost regardless of the type of alloplast or allograft material. This is in part because the antrum is surrounded by bone, and the primary source of revascularization of the graft comes from the adjacent bony walls.
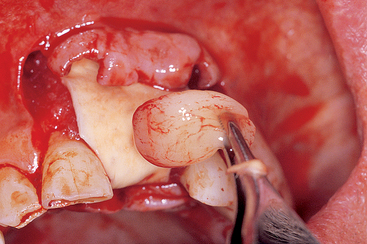
Blood vessels from bone that enter the graft site provide pluripotential perivascular cells that have the capability to become osteoblasts. Monocysts in the blood form osteoclasts, which precede the blood vessel into the bone graft site by forming cutting cones, which resorb devital bone and graft material. As the osteoclasts resorb the graft material, the blood vessel can grow into the site. As importantly, the sides of the blood vessel carry osteoblasts, but only when the vessel comes from the host bone (Figure 36-12). Not only is the blood vessel needed to help the autograft maintain vitality, it is also needed to repopulate the area with osteoblasts to grow new bone. It has been postulated that the surface fibroblasts, if left to migrate within the graft, not only invade the space, but also may inhibit osteogenesis by contact inhibition.56
A tooth extraction socket fills with bone because the blood vessels from bone form granulation tissue in the site and prevent the epithelial cells from migrating into the site. Four to 6 months are then needed for the socket to replace the area filled with the blood clot (initially) and granulation tissue (later) with bone.71
One effective method to increase the amount of host blood vessels in a graft site is to decorticate the host site with a rotary drill.72 The blood vessels from the trabecular bone are then able to invade the graft site from below and bring pluripotential cells. To permit blood vessels from the bone to enter the graft site, there should be spaces available between the graft particles. When autogenous bone is used as a graft material, the trabecular bone graft provides these open spaces, whereas cortical bone, which presents a denser surface, takes longer to revascularize. Spaces are also necessary when alloplasts are included in the bone graft site.
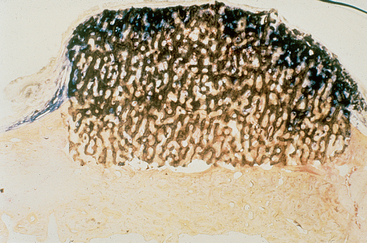
When an alloplast is placed upon a decorticated ridge and covered with soft tissue (without a barrier membrane), the bone from below the graft grows four times faster into the graft voids than the fibrous tissue grows down into the graft (Figure 36-13). This is because the blood vessels from bone grow rapidly into the region, and once they invade the site, bone follows.
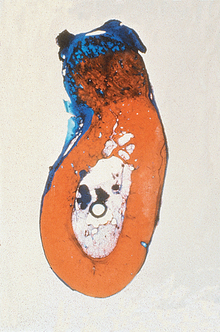
Figure 36-13 A bone graft site is decorticated and a “barrier by bulk” technique is used with an alloplast (in this case particulate hydroxyapatite) and covered with fibrous tissue. After 4 months, a block section was obtained and stained. The soft tissue is stained blue and the bone red. Note the bone grew up into the graft material four times farther than the soft tissue grew down into the graft. This is because the blood vessels grow faster than the fibrous tissue, and when the vessels come from bone, bone cells repopulate the graft and form bone. (note: The same mechanism occurred in Figure 36-8.)
An alloplast placed upon host cortical bone forms fibrous tissue around the graft, as no host bone blood vessels are able to grow into the graft material (Figure 36-14). The key to whether bone or fibrous tissue forms in the bone graft site is the host blood vessels.
Growth Factors
Bone growth factors can enhance formation and mineralization of bone, induce undifferentiated mesenchymal cells to differentiate into bone cells, and trigger a cascade of intracellular reactions and the release of a number of additional bone growth factors and cell-enhancing factors. These growth factors bind to specific receptors on the surface of target cells. More than 50 known growth factors have been identified and categorized, with some specific to the functions in bone healing (Box 36-1). These constitute a separate group of proteins because of the way they can be produced and their mode of action. They are, however, part of the large superfamily of TGF-β.73 Bone growth factors are primarily present in bone matrix and released during remodeling or after trauma. They act on the local osteoprogenitor differentiated cells and therefore have limited areas of action.74 In contrast, BMPs, although also found in extracellular bone matrix, are osteoinductive and can trigger the differentiation of mesenchymal cells into osteoblasts.75
Platelet-Derived Growth Factors
Platelet-derived growth factors are produced by activated macrophages and stored in platelets and bone matrix.76 Platelets are the greatest source of this growth factor and may be further divided into different types, AA, BB (homodimers), and AB (heterodimers). These factors have the characteristics of a wound hormone, acting as a chemoattractant and recruiting mesenchymal cells into the wound.77,78 The activated platelets also enhance hemostasis by attracting additional platelets to the site, which release thrombin, thromboxane A2, and adenosine diphosphate. Howes et al. used demineralized bone powder with PDGF in older rats to generate an increased production of mRNA for collagen II, alkaline phosphatase activity, and calcium content of the demineralized bone compared with controls without PDGF.79 PDGFs increased cartilage and bone formation in the graft site. They have also been shown to activate collagenase within the latter stages of wound healing, which remodels collagen to promote soft tissue wound healing.
The primary roles of PDGFs in bone modeling and remodeling are to (1) increase the number of cells necessary for bone formation (including osteoblasts) at the repair site; (2) trigger capillary formation through its potent mitogenic activity; (3) enhance site debridement; and (4) provide a continued source of growth factors for bone repairs.80 PDGF mixed with autologous bone grafts can accelerate mineralization by as much as 40% during the first year.79,81–89 PDGF combined with other growth factors (such as IGF)90 have shown promising results in periodontal regeneration with guided bone regeneration (GBR).91 Further research targets the use of PDGF combined with IGF to enhance the implant-bone interface.92
Fibroblast Growth Factors
Fibroblast growth factors are stored in the extracellular matrix of bone and have many of the same functions as PDGFs. FGFs can stimulate the proliferation of osteoblasts, resulting in the net formation of bone. In addition, they are a potent angiogenic factor.93 However, FGF-β needs to be in the presence of bone to be effective and is more effective when used in combination with PDGF. Research on the potential benefits of FGF is ongoing but has yielded varied results, with some studies showing bone abnormalities94 or no bone growth at all.95
Transforming Growth Factor Beta
The super family of growth factors is TGF-β, with more than 47 known varieties. TFG-β includes cytokines that contribute to connective tissue repair and bone regeneration. Bone is the body’s most abundant storage site for TGF-β, which acts as a weak mitogen for human osteoblastic cells.93 TGF-β also induces chemotaxis and stimulates extracellular matrix formation in osteoblastic cells and may inhibit osteoclast formation.93
TGF-β1 activates fibroblasts to form procollagen, which deposits type 1 collagen within the wound77,90 and is therefore credited with the enhancement of soft and hard tissue repair. PDGF and TGF-β1 assist in soft tissue healing and bone mineralization; therefore they can be mixed with grafting materials into the bone graft site and applied to the top layer of the graft.
Insulin-Like Growth Factors I and II
Insulin-like growth factors I and II (IGFI, II) mimic insulin in several ways. They are fabricated in the liver and travel through the blood stream. IGF has been shown to act as a chemotactic factor for mesenchymal progenitor cells derived from bone marrow.96
PRP is a volume of autogenous plasma that has a platelet concentration above baseline97 (1 million platelets/μL versus normal average of 200,000 platelets/μL). It is a concentrated autogenous source of seven growth factors that are “native growth factors in their biologically determined ratios”88 (contrary to recombinant growth factors, which are high concentrations of synthesized growth factors in the laboratory) (Box 36-2). It is not osteoinductive by itself, cannot induce bone formation alone, and therefore needs to be in the presence of bone or DFDB. However, it is a benefit to soft tissue healing also, and because soft tissue closure and blood supply are important keys to bone grafting, it is also a secondary benefit even when autologous or DFDB bone is not present.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses