Chapter 3
Dentinal and pulpal pain
Introduction
The dental pulp is exceptionally richly innervated by tri-geminal afferent axons (7, 10) that seem to subserve mostly, if not exclusively, nociceptive function (30, 39, 43). Accordingly, they respond to stimuli that induce or threaten to induce injury to the pulp tissue, and their activation may induce defensive, withdrawal-type reflexes in the masticatory muscles (37, 43, 49). The pain responses induced by external stimuli can be extremely intense. The dense innervation of the pulp and dentin gives a morphological basis for the high sensitivity of these tissues. In addition to the afferent sensory nerves, the dental pulp is innervated by autonomic sympathetic efferents that play a role in the regulation of the blood flow in the pulp (47) and, in addition, may have regulatory functions in pulpal inflammation (24, 54). The existence and functional significance of parasympathetic innervation are still controversial (47).
Classification of nerve fibers
Nerves can be divided into different groups according to axon size and structure, which determine the conduction velocities of the individual fibers (22) (Table 3.1). In the nervous system the different-sized fibers are distributed in a functionally meaningful manner, namely thick myelinated fibers in those nerve tracts where fast conduction is demanded and fine-caliber fibers in those tracts where the speed of conduction is not as critical. For example, the efferent Aα-motoneurons, which transmit nerve impulses to the skeletal muscles, have thick myelinated axons and conduction velocities of up to 120 m/s (22). The afferent Aβ-type sensory axons (with conduction velocities of 30–70 m/s) transmit touch and pressure sensations and, usually, their receptors respond to light mechanical forces, i.e. they have low stimulation thresholds (22).
Table 3.1 Examples of different nerve fiber types, their functions, diameters and conduction velocities.
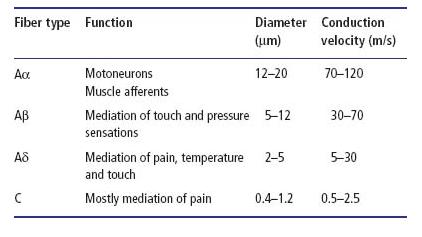
Pain is conducted by two different sets of neurons: thin myelinated Aδ-fibers with conduction velocities of 5–30 m/s and neurons with unmyelinated axons with conduction velocities of 0.5–2.5 m/s. Because of this organization, the sensation perceived in response to noxious stimulation consists of two discrete and different components: first sharp and rather well-localized pain mediated by Aδ-fibers and then delayed, dull pain that is mediated by C-fibers and can radiate to a wide area surrounding the affected tissue (39). The poor localization of the C-fiber pain is due to more extensive convergence of the afferent C-fibers, compared to A-fibers, to the second-order neurons in the brain stem (22). Under experimental conditions, the temporal discrimination and the quality differences of the two pain components can be demonstrated clearly in response to stimulation of extremities (39). The same dichotomy in the quality of pain can be shown when stimulating teeth (30), although temporal discrimination is not as obvious because of the short distance between the brain and the site of stimulation.
Morphology of intradental sensory innervation
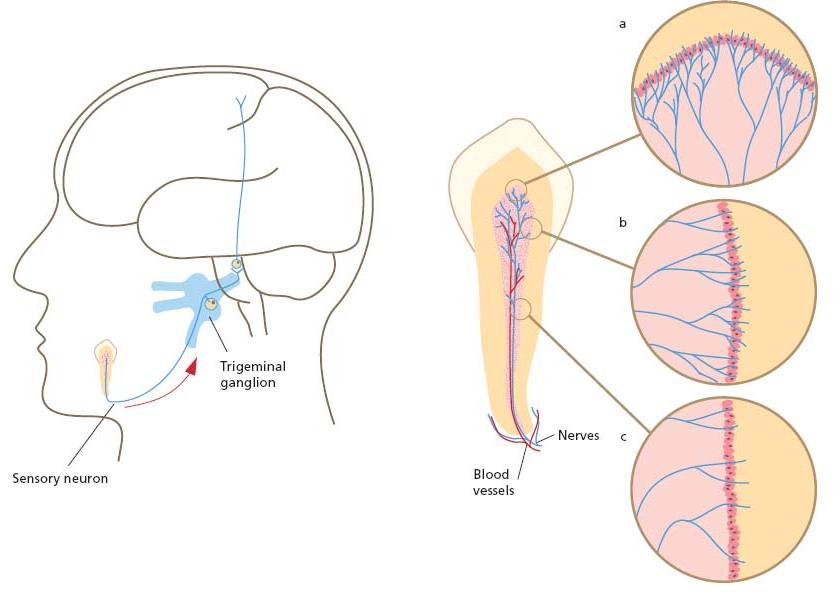
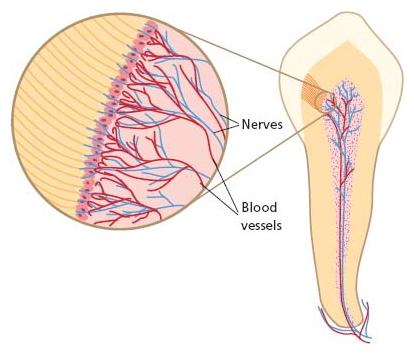
The sensory neurons of the dental pulp have their cell bodies in the trigeminal ganglion (7, 10). The teeth of the upper jaw are innervated by neurons of the maxillary and those of the lower jaw by neurons of the mandibular division of the trigeminal nerve. The pulpal axons are located in the alveolar branches of the nerve and finally enter the pulp through the apical foramen or multiple foramina of the root apex in close proximity to the intradental blood vessels (Fig. 3.1).
Fig. 3.1 Schematic drawing presenting the innervation of the dental pulp. Several branches from the alveolar nerve enter the apical area of the tooth. Some nerve bundles innervate the periodontal tissues. Multiple bundles enter the pulp in close proximity to the blood vessels through the apical foramen; they branch further on their way to the tooth crown. Most of the intradental axons have their terminals in the pulp/dentin border of the coronal pulp, which is the most densely innervated area in the tissue (a). There are fewer nerve endings in the cervical area (b) and the pulp/dentin border in the root pulp is sparsely innervated (c).
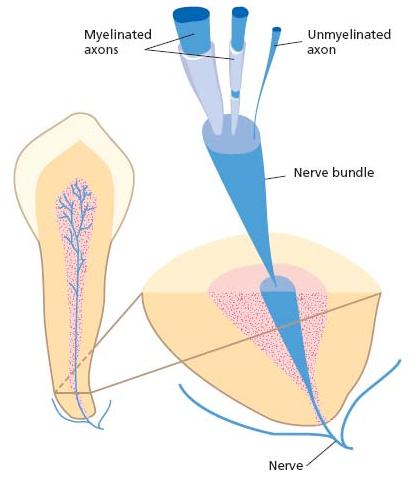
Several hundred axons per tooth enter the pulp at the root apex; for premolars, this number is close to a thousand (7, 26, 29). The nerve fibers enter the tooth pulp in multiple bundles that contain both myelinated and unmyelinated axons (Fig. 3.2) (7, 26, 29). The majority of the axons (70–80%) are unmyelinated (7, 26, 29).
In species as varied as human, cat, dog, monkey and ferret, it appears that there are no gross differences in intradental innervation (7, 10). Rat molars also have similar innervation but in the incisors, which are continuously erupting, the innervation is sparse and of a different structure. For example, these teeth lack dentinal nerve fibers (7, 10).
Only a small proportion of the pulpal afferents terminate in the root. Most of the nerve bundles extend to the coronal pulp, branching on their way (Fig. 3.1). The terminal branch endings are located mostly in the pulp/dentin border area of the coronal pulp (Fig. 3.3). A dense network of fine nerve filaments, known as the nerve plexus of Raschkow, is formed close to the odontoblasts. Several nerve terminals also enter the odontoblast layer and many of them extend into the dentinal tubules (7, 10) (Fig. 3.3). Both morphological and functional studies indicate that the fine nerve filaments in the dentinal tubules are mostly terminals of the myelinated intradental axons (7, 10, 40, 43). Some of the axons also terminate in the deeper parts of the pulp, often in close proximity to the pulpal blood vessels, and they may have a significant role in the mediation of pulpal blood flow responses to external irritation, as well as in pulp tissue inflammation and repair (9, 10, 47, 48; see also Chapter 2).
The terminal branching of the pulpal nerve fibers is extensive (7). Individual myelinated axons may innervate more than 100 dentinal tubules. Accordingly, innervation of the pulp/dentin border is extremely dense. Both myelinated and unmyelinated fibers terminate as free nerve endings. These are the receptors or nociceptors, which respond to various external stimuli in normal teeth and to the environmental changes and various inflammatory mediators that occur under pathological conditions.
Fig. 3.2 A schematic drawing showing a nerve bundle entering the pulp chamber in the apical area of the tooth. The nerve bundle contains both un myelinated and myelinated axons of variable sizes.
Fig. 3.3 Innervation of the pulp/dentin border in the coronal pulp. The nerve fibers entering the area form a dense network known as the plexus of Raschkow. The fibers form free nerve endings in the peripheral pulp and in the odontoblast layer. Many nerve terminals are also located in the dentinal tubules. Some fibers branch to innervate the adjacent blood vessels (see text for details).
As in other tissues, the sensory nerves of the dental pulp contain neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) (8–12). Several different neuropeptides have been identified in various parts of the nervous system (18, 34) that act as neuro-mediators or modulators and have significant regulatory effects on impulse transmission in the central nervous system. Many of them have also been shown to function in peripheral tissues as, for example, mediator substances in the effector organs of the autonomic sympathetic and parasympathetic nerves (34). The sensory neuropeptides in the afferent nerves play an important role in the initial stages of the inflammatory process (neurogenic inflammation) following injury in the peripheral tissues (47, 48) and also seem to regulate the later stages of inflammation and repair (8–12).
The location of the nerve terminals in dentin is limited to the inner 150–200 μm of the tubules (7, 10). The outer layers of dentin are not innervated. It should also be noted that innervation of the dentin is densest in the coronal part, especially in the pulp tips under the cusps, where about 50% of the tubules have been shown to contain nerve fibers (7). Many tubules contain several nerve endings (7, 28). Considering the structural dimensions of dentin it can be estimated that approximately 15 000–20 000 nerve endings/mm2 exist in the pulp/dentin border area at the pulp tip. Innervation of the pulp/dentin border becomes less dense towards the cervical areas and the number of innervated tubules becomes considerably lower (7, 10). In addition, the distance that the nerve fibers penetrate into the tubules is much shorter than in the coronal areas. In the root, the innervation of the peripheral pulp and dentin is sparse (7). In this respect the structural organization of intradental innervation seems to be poorly correlated to the sensitivity of different dentin areas in the clinical situation, namely, that exposed cervical dentin seems to be especially sensitive. However, this obvious discrepancy can be due to differences in the time the dentin has been exposed and in the responses of the pulp–dentin complex to irritation in the coronal dentin compared with the cervical dentin (see section on dentin hypersensitivity below). The variation in dentinal innervation in different parts of the tooth may explain the different types of pain response induced in the coronal versus the root dentin in human teeth (32).
Some afferent nerve fibers may branch to innervate both the dental pulp and the adjacent tissues or multiple teeth. Such organization may, to some extent, contribute to the poor localization of dental pain and may also allow neurogenic vasodilation and inflammatory reactions to occur in an area of tissue wider than that affected by the original insult. Correspondingly, within the dental pulp the terminal branching of the nerve fibers may contribute to the spread of the inflammatory reactions (44).
Function of intradental sensory nerves under normal conditions
Knowledge of the function of intradental nerves is mostly based on electrophysiological recordings performed on experimental animals (40, 43, 44, 46). Comparison of the nerve responses to the sensations induced from human teeth with the same stimuli, as well as to the clinical cases of dental pain, has given insight to the contribution of the different intradental nerve fiber groups to different dental pain sensations. The apparently similar structure and function of the innervation in the different species examined (human, monkey, dog, cat and ferret) gives a reasonable basis for such comparisons (Advanced concept 3.1).
As already mentioned, the pulp and dentin are innervated by two different groups of afferents: A- and C-fibers (30, 40, 43). The functional classification is based on the conduction velocities of the axons and corresponds to the morphological findings showing the existence of both myelinated and unmyelinated nerve fibers in the pulp (7, 26,29).
Intradental A- and C-fibers are functionally different (10, 30, 43, 44). The A-fibers respond to various “hydro-dynamic” stimuli applied to dentin, such as drilling, probing, air-drying and hypertonic chemical solutions (40, 42, 43). There seems to be a common mechanism of nerve fiber activation in response to the different stimuli. This hydrodynamic mechanism will be described and discussed in detail later in this chapter. The pulpal C-fibers are polymodal, which means that they respond to several different stimuli when they reach the pulp proper (30, 40, 43). The fibers have high thresholds and are activated by intense thermal (heat and cold) and mechanical stimulation (30, 40, 43). They also respond to such inflammatory mediators as bradykinin and hista-mine (40, 43), which are both formed and/or released in response to tissue injury and associated inflammatory reactions. Thus, the results from the electrophysiological studies indicate that intradental A-fibers are responsible for the sensitivity of dentin and may give the first warning signals whenever dentin is exposed, whereas the C-fibers may be activated mostly under pathological conditions.
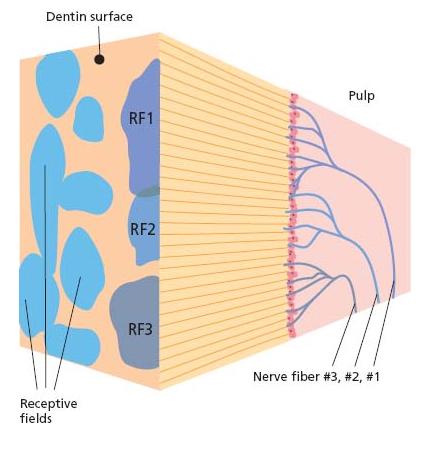
Discrete receptive fields of the intradental nerve fibers can be located in either the pulp or dentin (30, 40, 44, 62). The receptive fields of C-fibers are found in the pulp proper and the pulp tissue has to be exposed for their activation. Also, some A-fibers, mostly slowly conducting, have their receptive fields in the pulp and thus cannot be activated by dentinal stimulation (44, 62). On the other hand, the receptive fields of those A-fibers that are activated by hydrodynamic stimulation of dentin can be located by probing the exposed dentin surface (44, 62). In normal teeth the receptive fields are usually small spots a few millimeters in diameter (Fig. 3.4). Some fibers may have two or even three separate receptive fields that can be located at a considerable distance from each other, in the coronal dentin and cervical area in some cases (62). Typically, the receptive fields of individual fibers overlap extensively, meaning that stimulation of a small area in dentin or pulp can activate multiple nerve fibers; this is an important factor, considering the intensity of the pain responses induced by external stimulation. These functional findings are in accordance with the structure of innervation of the pulp/dentin border area, with the extensive and overlapping terminal branching of the individual axons (see above) (Fig. 3.4).
The intradental C-fibers are activated by a direct effect of the applied stimuli on the nerve endings (30, 40, 43). For example, in thermal stimulation the response latencies are rather long and nerve firing does not begin until the temperature within the pulp has changed by several degrees centigrade (30, 40). Similarly, activation of the most slowly conducting Aδ-fibers seems to result from a direct effect of the stimuli on the nerve endings (44, 62). It also seems that the pulpal C-fibers and slowly conducting Aδ-fibers are “silent” in normal healthy teeth and may become active only in cases of pulp injury and inflammation. On the contrary, the A-fibers (mostly faster conducting) responsible for the sensitivity of dentin respond readily whenever dentin is exposed. The nerve activation is immediate or of a very short latency compared with C-fibers, which is in accordance with their activation mechanism (see below).
Fig. 3.4 Schematic drawing showing the receptive fields of ten individual pulp nerve fibers on the exposed dentin surface. The receptive fields are of variable shape and overlapping, and in a normal tooth are rather small. The terminal branching of three nerve fibers (fibers 1, 2 and 3) in the pulp/dentin border on the right side of the figure and the corresponding receptive fields (RF1, RF2 and RF3) on the dentin surface are shown as examples. The RF of each individual fiber corresponds to the area in the pulp/dentin border innervated by the particular axon and connected to the receptive field on the dentin surface by the dentinal tubules.
Comparison of the above-described nerve responses to the pain sensations induced from human teeth under similar stimulation conditions has revealed how different intradental nerve fiber groups may contribute to the different dental pain conditions. For example, “hydrody-namic” stimuli, which activate only pulpal A-fibers, induce sharp pain when applied to dentin in human subjects (42, 43). Intense thermal stimulation of human teeth has been shown to induce an initial sharp pain sensation followed by delayed dull and lingering pain if the stimulation is continued (30). Similar stimulation of the cat canine tooth induces a brief, short latency firing of intradental A-fibers followed by a long latency activation of C-fibers (30, 40, 43). Consequently algogenic (pain-producing) agents, which activate selectively either A- or C-fibers in experimental animals (40, 43), induce sharp or dull and lingering pain in human subjects (2). Altogether, the above results indicate that intradental A-fibers mediate the sharp dental pain sensations and are responsible for dentin sensitivity, whereas C-fibers mediate the dull pulpal pain or toothache connected with pulpitis.
In spite of the type of stimulus applied to a tooth, pain is the only sensation induced in response to activation of the pulpal sensory nerves, according to most studies. The only exception is low-intensity electrical stimulation (39, 43), which can induce so-called prepain sensations that probably result from low-level (liminal) activity in the pulpal nociceptive afferents. Considering any clinical situations when an electric pulp tester is used, it is important to note that the initial sensation at threshold level is usually not painful. When the stimulus intensity is increased, the sensation becomes painful and any other stimulus applied to the tooth induces pain, although its quality may vary in response to different stimuli. The variation is due to differences in the nerve response patterns and the activation of different types of nerve fibers (35).
Sensitivity of dentin: hydrodynamic mechanism in pulpal A-fiber activation
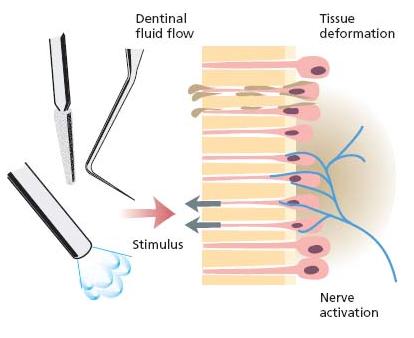
The “hydrodynamic hypothesis” explaining the sensitivity of dentin was first presented by Gysi (23) in 1900. Today it is widely accepted that the sensitivity of dentin is based on hydrodynamic activation of the intradental A-fibers (Fig. 3.5). This concept is supported by a considerable amount of in vitro and in vivo data from both human and animal experiments (5, 6, 40, 42, 44). It was shown in the early 1960s and in a number of later studies that stimuli inducing pain, when applied to human dentin, are able to induce fluid flow in dentinal tubules in vitro (5, 6). The strong capillary forces in the fine tubules cause the hydrodynamic fluid flow. In general, desiccating or evaporative stimuli are the most effective because the capillary force contributes to the outward movements of the tubule contents. It is much more difficult to induce inward fluid flow (6, 50). Moreover, the nerve fibers seem to be more sensitive to outward than inward fluid movements (59). The fluid flow causes mechanical distortion of the tissue in the pulp/dentin border area where most of the nerve endings are located (Figs 3.1 and 3.3–3.5). Accordingly, with all hydrody-namic stimuli the final factor inducing activation of the nerve endings or receptors is a mechanical effect. The results from single pulp nerve recordings showing that individual nerve fibers respond to several different hydrodynamic stimuli are in line with this concept (40, 43, 44).
Fig. 3.5 The hydrodynamic mechanism of pulp nerve activation. Any stimulus capable of removing fluid from the outer ends of the dentinal tubules activates hydrodynamic fluid movement. The lost fluid is replaced by an immediate outward flow due to the high capillary forces in the dentinal tubules. The fluid flow causes mechanical distortion of the tissue with the nerve endings in the pulp/dentin border.
The fluid flow in the dentinal tubules must be rapid enough to induce sufficient mechanical effect for activation of the nerve endings in the pulp/dentin border. Although there is continuous, slow outward flow in the tubules of exposed dentin due to the high capillary and tissue fluid pressure in the pulp, such a flow is not sufficient to cause nerve activation (50, 58, 59). As already me/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses