Chapter 21 Pharmacology in Implant Dentistry
Because of the increase in demand and use of dental implants in dentistry today, a thorough understanding of the indications and protocol for the use of pharmacologic agents in implant dentistry is essential. The morbidity of implant-related complications may on occasion be significant; therefore indicated drug selection and sufficient dosage levels of medications are preoperatively and postoperatively indicated. The scope of implant treatment often encompasses an older population with more complex cases. As a result, a greater understanding of compromised wound healing and inadequate immune systems with respect to pharmacology is essential.
ANTIMICROBIALS
Antibiotics
Prophylactic Antibiotics
In general surgery, including its subspecialties, principles of antibiotic prophylaxis are well established. Guidelines are specific related to the procedure, the type of antibiotic, and the dosage regimen.1,2 The use of prophylactic antibiotics in dentistry has also been documented in the prevention of complications for patients at risk of developing infectious endocarditis and immunocompromised patients. However, in oral implantology, there exists no consensus on the use and indications for prophylactic antibiotics. Disadvantages with the use of antibiotics include development of resistant bacteria, adverse reactions and possible resultant lax surgical technique.3–5 As a result, the need for prophylactic antibiotics in healthy patients, type of antibiotic, dosage, and duration of coverage is controversial. On the other hand, postoperative surgical wound infections can have a significant impact on the well-being of the patient and the survival of the implant. Documented cases of potential consequences of infection range from increased pain and edema to even patient death. According to Esposito and Hirsch, one of the main causes of dental implant failure may be due to bacterial contamination at implant insertion.6
A local inoculum must be present for a surgical wound infection to occur, to overcome the host’s defenses and allow growth of the bacteria. This process has many variables including various host, local tissue, and systemic and microbial virulence factors. Antibiotic prophylaxis is only one component of this complex cascade; however, the efficacy and impact of antimicrobial prophylaxis has been proven to be significant.3
Several studies have concluded there is benefit of preoperative antibiotics for dental implantology.7–9 In the most comprehensive and controlled study to date, 33 hospitals formed the Dental Implant Clinical Research Group and concluded that the use of preoperative antibiotics significantly improved dental implant survival, both in early and later stages. In the evaluation of 2973 implants, a significant difference was found with the use of preoperative antibiotics (4.6% failure) compared with no antibiotics (10% failure).7,8
The main goal of the use of prophylactic antibiotics is to prevent infection during the initial healing period from the surgical wound site, thus decreasing the risk of infectious complications of the soft and hard tissues. Although there is no conclusive evidence on the mechanism of preoperative antibiotics, most likely a greater aseptic local environment is achieved. A landmark study by Burke defined the scientific basis for the perioperative use of antibiotics to prevent surgical wound infection.10 From this work, several accepted principles have been established in the perioperative use of prophylactic antibiotics.11
Principle 1: The Procedure Should Have a Significant Risk for and Incidence of Postoperative Infection
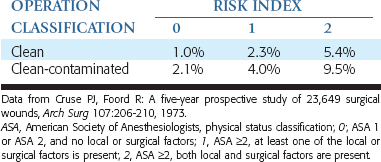
To evaluate the risk for postoperative wound infection, a classification of operative wounds and risk of infection was developed by the American College of Surgeons (Committee on Control of Surgical Wound Infections). All surgical procedures were classified according to four levels of contamination and infection rates (Box 21-1). Within these classifications, it is generally accepted that all class 2, class 3, and class 4 procedures warrant the use of prophylactic antibiotics.12
Box 21-1 Surgical Wound Classifications with Associated Infection Rates
By definition, elective dental implant surgery falls within the class 2 (clean-contaminated) category. Class 2 medical and dental surgical procedures have been shown to have an infection rate of 10% to 15%. However, with proper surgical technique and prophylactic antibiotics, the incidence of infection may be reduced to less than 1%.13,14 In a healthy patient, risk of infection after dental implant surgery is influenced by numerous factors such as type and location of surgery, skill of the surgeon, methods of intraoperative management, patient factors, and aseptic technique.13,15 Moreover, additional patient-related (systemic and local) risk factors that are not addressed in these classifications have also been correlated with increased susceptibility to infection. These factors must be addressed in reference to evaluation for the use and duration of antibiotic prophylaxis (Box 21-2).
Box 21-2 Factors Associated with Increased Risk of Infection for Dental Implant Procedures
ASA, American Society of Anesthesiologists, physical status classification.
One of the most significant surgical factors that may contribute to infection is poor aseptic technique. Various routes of transmission of virulent bacteria include (1) direct contact with the patient’s blood or other body fluids; (2) indirect contact with contaminated objects; (3) contact of infected nasal, sinus, or oral mucosa; and (4) inhalation of airborne microorganisms. To prevent these conditions, a controlled, well-monitored aseptic setting should be achieved for the surgical procedure. The aseptic surgical site includes proper disinfection and draping procedures of the patient, hand scrubbing, sterile gowns worn by all surgical members, and maintenance of complete sterility of the instrumentation.
Another important surgical factor related to postoperative infection is the duration of the surgical procedure. This factor has been shown to be the second most critical risk factor (after wound contamination) affecting postoperative infection rates.18 In general, surgical operations lasting less than 1 hour have an infection rate of 1.3%, whereas those lasting 3 hours increase the rate to more than 4%.19–21 It is postulated that the rate of infection doubles with every hour of the procedure.16
The skill and the experience of the surgeon with the placement of implants have been shown to be significant in postoperative infections and implant failures. A recent study has shown that less experienced surgeons (<50 implants placed) have a 7.3% increase in failure rates in comparison to experienced surgeons.8
In the medical literature, it is well documented that the insertion of any prosthetic implant or device increases the chance of infection at the surgical site. A dental implant can act as a foreign body, and the host’s defenses may therefore be compromised. The surface of the implant has been shown to facilitate bacterial adherence and the presence of an implant can compromise the host’s defenses. This may result in normal flora with low virulence potential to cause infections at the implant-host interface, which has been shown to be very difficult to treat.22–24
The probability of risk for infection for a given procedure is related to local, systemic, and surgical factors. The patient’s American Society of Anesthesiologists (ASA) score may be used as the systemic factor and then correlated with various local and surgical factors. A risk index may then be modified from the literature to correlate these factors to dental implant surgeries (Table 21-1). The probability of wound infection may then be correlated with the type of wound contamination (class 1 to 4) and the risk index. Therefore a class 2 wound and a risk index 2 has a greater risk of complications, and a class 1 wound and risk index 0 has the least risk of postoperative infection.19,25
Principle 2: The Appropriate Antibiotic for the Surgical Procedure Must Be Selected
The prophylactic antibiotic should be effective against the bacteria that are most likely to cause an infection. In the majority of cases, infections after surgery are from organisms that originate from the site of surgery.11 Most postoperative infections are caused by endogenous bacteria including aerobic gram-positive cocci (streptococci), anaerobic gram-positive cocci (peptococci), and anaerobic gram-negative rods (bacteroides)13 (Box 21-3).
Although oral infections are mixed infections in which anaerobes outnumber aerobes 2:1, it has been shown that anaerobes need the aerobes to provide an environment to proliferate.26 Subsequent studies have shown that the early phase of intraoral infections involve streptococci that prepare the environment for subsequent anaerobic invasion.27,28 With that in mind, the ideal antibiotic must be effective against these pathogens.
The final selection factor is that the antibiotic should ideally be bactericidal. The goal of antibiotic prophylaxis is to kill and destroy the bacteria. Bacteriostatic antibiotics work by inhibiting growth and reproduction of bacteria, thus allowing the host defenses to eliminate the resultant bacteria. However, if the host’s defenses are compromised in any way, the bacteria and infection may flourish. Bactericidal antibiotics are advantageous over bacteriostatic antibiotics in that (1) there is less reliance on host resistance, (2) the bacteria may be destroyed by the antibiotic alone, (3) results are faster than with bacteriostatic medications, and (4) there is greater flexibility with dosage intervals.13
Principle 3: An Appropriate Tissue Concentration of the Antibiotic Must Be Present at the Time of Surgery
For an antibiotic to be effective, a sufficient tissue concentration must be present at the time of bacterial invasion. To accomplish this goal, the antibiotic should be given in a dose that will reach plasma levels that are three to four times the minimum inhibitory concentration of the expected bacteria.29 The minimum inhibitory concentration is the lowest antibiotic concentration to destroy the specific bacteria. Usually, to achieve this cellular level, the antibiotic must be given at twice the therapeutic dose and at least 1 hour before surgery.15 It has been shown that normal therapeutic blood levels are ineffective to counteract bacterial invasion. If antibiotic administration occurs after bacterial contamination, no preventive influence has been seen as compared with taking no preoperative antibiotic.
Principle 4: Use of the Shortest Effective Antibiotic
In a healthy patient, continuing antibiotics after surgery often does not decrease the incidence of surgical wound infections.2,30,31 In a healthy patient, a single dose of antibiotics is usually sufficient. However, for patients or procedures with increased risk factors (see Box 21-2), a longer dose of antibiotics is warranted.11 With the high degree of morbidity associated with dental implant infections, one must weigh the benefits versus risk involved for the extended use of antibiotics.
Complications of Antibiotic Prophylaxis
It is estimated that approximately 6% to 7% of patients taking antibiotics will have some type of adverse event.32 Incidence of significant complications with the use of prophylactic antibiotics are minimal; however, a small percentage can be life-threatening. The risks associated with antibiotics include gastrointestinal (GI) tract complications, colonization of resistant or fungal strains, crossreactions with other medications, and allergic reactions.
Allergic reactions have a wide range of complications, ranging from mild urticaria to an anaphylaxis and death. Studies have shown that 1% to 3% of the population receiving penicillin will exhibit urticaria type of reactions, with 0.04% to 0.011% having true anaphylactic episodes. Of this small percentage of anaphylactic reactions, 10% will be fatal.33
An unusual but increasing complication in the general population after antibiotic use is pseudomembranous colitis. This condition is caused by the intestinal flora being altered and colonized by Clostridium difficile. Penicillin and clindamycin use has been significantly associated with pseudomembranous colitis; however, all antibiotics have been shown as potential causative agents. The risk levels of colitis related to antibiotics are outlined in Table 21-2. The most common treatment for antibiotic- induced colitis is vancomycin or metronidazole.
Table 21-2 Risks for Antibiotic-Induced Pseudomembranous Colitis
| HIGH | MEDIUM | LOW |
|---|---|---|
The most recent concern of antibiotic use is the development of resistant bacteria. It has been observed that the overgrowth of resistant bacteria begins only after the host’s susceptible bacteria are killed, which usually takes at least 3 days of antibiotic use. Therefore short-term (1 day) use of antibiotics has been shown to have little influence on the growth of resistant bacteria.13
Antibiotics Used in Implant Dentistry
Beta-Lactam Antibiotics
Amoxicillin/Clavulanic Acid (Augmentin)
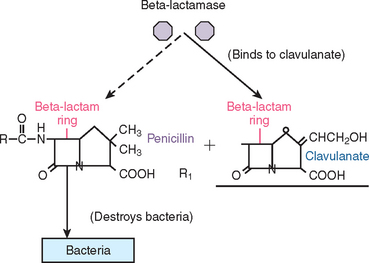
To counteract the activity of beta-lactamase destruction of penicillins by resistant bacteria such as Streptococcus aureus, a combination of two antibiotics was synthesized. Clavulanic acid, a beta-lactam antibiotic, was added to amoxicillin to form Augmentin. This combination antibiotic has an affinity for penicillinase-producing bacteria. It functions as a “suicide molecule” that inactivates the resistant bacteria. As a result of an increase in the prevalence of these specific bacteria (especially in the sinus), Augmentin is becoming more popular in oral implantology. This antibiotic is used mainly in cases in which penicillinase bacteria is suspected (or known by culture) and is very practical as a perioperative antibiotic for sinus augmentation (Figure 21-1).
Cephalexin/Cefadroxil
The first-generation cephalexin/cefadroxil antibiotics have an antibacterial spectrum similar to amoxicillin. However, they have the advantage of not being susceptible to beta-lactamase destruction by S. aureus. They are often used in dentistry as an alternative for the penicillin-allergic patient, although cross-reactivity between these two drugs may occur. Rates of cross-reactivity to first-generation cephalosporins with penicillin-allergic patients have been cited to be approximately 8% to 18%. The most recent studies have shown only patients who have had type I (immunoglobulin E: immediate hypersensitivity reactions) should not be administered a cephalosporin. If the patient has a previous history of a reaction that was not immunoglobulin E–mediated (types II, III, IV, or idiopathic reactions) a first-generation cephalosporin may be administered. Newer second- and third-generation cephalosporins exhibit a broader spectrum, less cross-reactivity, and a greater resistance to beta-lactamase destruction.34
Metronidazole
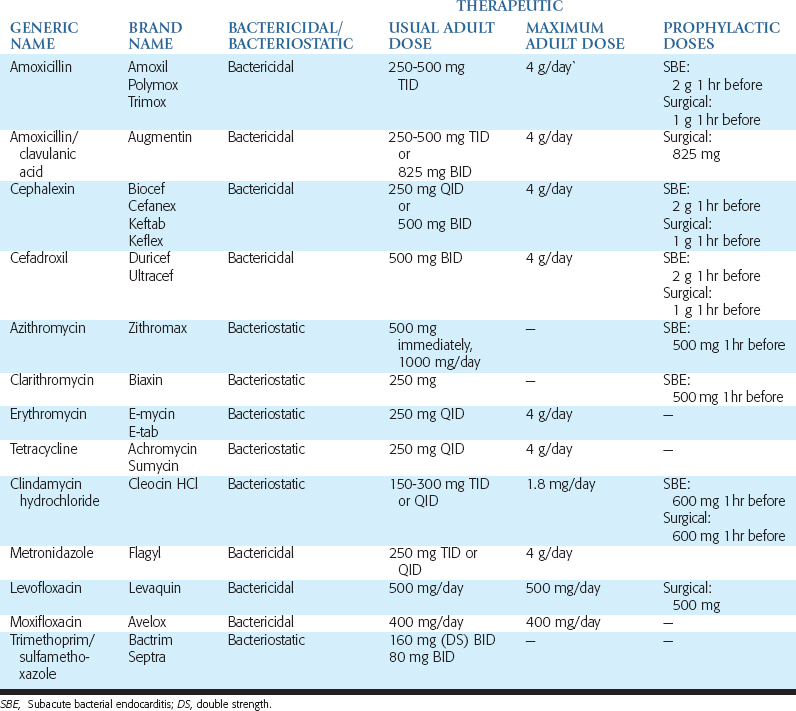
Metronidazole is a bacteriocidal antibiotic that is most often used for anaerobic infections. Because metronidazole has no activity against aerobic bacteria, it is seldom used for mixed infections unless it is combined with another antibiotic. However, it may be combined with penicillin when managing severe infections. Patients should be cautioned against drinking alcoholic beverages while taking this medication, because disulfiram-like reactions have been reported. These consist of severe nausea and abdominal cramping caused by the formation of a toxic compound resembling formaldehyde. Metronidazole should not be prescribed for patients taking the oral anticoagulant warfarin (Coumadin). The more common antibiotics and dosages used in oral implantology for prophylaxis, grafting and implant insertion, postoperative infection, and long-term complications are listed in Table 21-3.
THERAPEUTIC USE OF ANTIBIOTICS: POSTOPERATIVE INFECTIONS
Acute postoperative infections have been shown to occur on the third to fourth day after surgery. The most common microorganisms associated with peri-implant, postoperative complications have been previously stated as listed in Box 21-3.
Local signs of infection are pain, inflammation, bleeding, and exudate at the site of surgery. Systemic signs include fever, headache, nausea, muscle aches, vomiting, and weakness. When surgical wound infections arise, a specific diagnosis is advantageous to treat the complication. When evaluating the various antibiotics possible that are effective against the bacteria in question, a broad-spectrum beta-lactam antibiotic is most often the first-line medication. The duration of treatment should include antibiotic administration for 3 days beyond the occurrence of significant clinical improvement, usually at the fourth day and therefore for a minimum of 7 days.35
Therapeutic Antibiotics in Implant Dentistry
Amoxicillin (500 mg): Two immediately, then one tablet three times daily for 1 week
or if penicillin allergy exists
Clindamycin (300 mg): Two immediately, then one tablet three times daily for 1 week
Until culture and sensitivity test results are obtained, change antibiotic to:
Chlorhexidine
Another modality for antimicrobial prophylaxis for implant surgery is the use of an oral rinse, 0.12% chlorhexidine digluconate (Peridex; Procter & Gamble, Cincinnati, Ohio). Chlorhexidine gluconate is a potent antibacterial that causes lysis by binding to bacterial cell membranes. It has high substantivity that allows it, at high concentrations, to exhibit bacteriocidal qualities by causing bacterial cytoplasm precipitation and cell death.36,37 In the oral cavity, chlorhexidine has been shown to have a slow release from tissue surfaces over a 12-hour period.38,39
In vitro studies have shown an inhibitory effect of chlorhexidine on cultured epithelium and cell growth; however, clinical studies have not shown this effect.40,41,44 To the contrary, the use of chlorhexidine has been shown to be an effective adjuvant in reducing plaque accumulation, enhance mucosal health,42–44 improve soft tissue healing,45,46 treat periodontal disease, prevent alveolar osteitis,47,/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses