Chapter 21
Odontogenic and Non-odontogenic Tumors of the Jaws
Introduction
Odontogenic and non-odontogenic tumors of the jaws are a relatively rare and heterogeneous group of benign and malignant neoplasms, hamartomas, and other bone-related lesions that demonstrate great variability in etiology, biologic behavior, and clinical significance. The single commonality amongst these cellular proliferations is that they may occur in the jaws. Odontogenic tumors, by definition, are derived from tooth-related tissues and, although they have been reported in tooth-bearing structures such as dermoid cysts and teratomas, they originate overwhelmingly in the maxilla or mandible. The majority of these disorders are benign, and while they may be locally aggressive, only very rarely do they have the ability to metastasize. Non-odontogenic jaw tumors, on the other hand, develop from the epithelium and/or mesenchyme of a wide variety of tissues in the body, often originate in non-tooth-bearing facial bones, and may develop in other sites outside of the head and neck. Some of these are of little clinical significance and require nothing more than observation or local excision, while many of the malignant variants mandate multimodal therapy and portend a poor prognosis for survival.
Odontogenic Tumors
Odontogenic tumors (OT) are derived from tooth-forming elements and for the purposes of this chapter include lesions that are not definitively neoplastic. Even using this broad definition, OTs are rare lesions that account for <2–3% of all oral and maxillofacial specimens sent for diagnosis to oral pathology services.
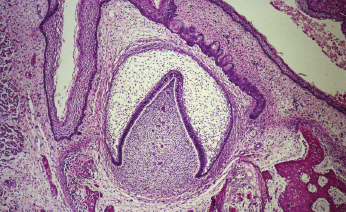
Odontogenesis occurs through a complex process involving the enamel organ, the dental follicle, and the dental papilla (Fig. 21.1). The enamel organ is an epithelial structure that is derived from oral ectoderm. The dental follicle and dental papilla are derived from neural crest cells and are therefore considered ectomesenchymal in nature. Odontogenic tumors demonstrate varying inductive interactions between the odontogenic epithelium and odontogenic ectomesenchyme, and are typically subclassified by their tissue of origin.
Classification
The current WHO classification of odontogenic tumors was published in July 2005 (Table 21.1) and made a number of important changes to terminology, benign or malignant classifications, and assignment to relevant subgroups. Previous WHO classifications described only tumor histology.
Table 21.1 WHO histological classification of odontogenic tumors. Adapted from Barnes L, Eveson JW, Reichart P, Sidransky D (2005) World Health Organization Classification of Tumours, Pathology and Genetics of Head and Neck Tumours. pp, 284. IARC Press, Lyon.
| Malignant tumors |
|---|
| Odontogenic carcinomas |
|
| Benign tumors |
| Odontogenic epithelium with mature, fibrous stroma without odontogenic ectomesenchyme |
|
| Odontogenic epithelium with odontogenic ectomesenchyme, with or without hard tissue formation |
|
| Mesenchyme and/or odontogenic ectomesenchyme with or without odontogenic epithelium |
|
| Bone-related lesions |
|
Odontogenic cysts were not considered in the new 2005 classification, which means that the previous 1992 classification is still relevant for cysts of the jaws and maxillofacial region, with one notable exception: the odontogenic keratocyst (OKC) was renamed “keratocystic odontogenic tumor” (KOT or KCOT) and was added to the odontogenic tumors category. The change in terminology is a reflection of contemporary clinical, molecular, and immunohistochemical data suggesting that the KOT is a benign neoplasm rather than a cyst.
Another important change in the 2005 WHO classification is related to malignant tumors of odontogenic origin. The metastasizing ameloblastoma was incorrectly described in the previous 1992 classification. It is now clarified in the 2005 document, which provides a clear distinction between the metastasizing ameloblastoma (containing benign histological features) and ameloblastic carcinoma. The diagnosis of an ameloblastic carcinoma is made in the presence of malignant histological features. In addition, the clear cell odontogenic carcinoma (CCOC), formerly clear cell odontogenic tumor, has been added to the list of malignant odontogenic carcinomas due to its newly recognized aggressive behavior.
Treatment Options for Benign Tumors of the Jaws
Enucleation and Curettage
Enucleation and curettage has been the traditional and time-honored method for managing odontogenic cysts and some jaw tumors. The technique offers the patient a minimally invasive procedure, with little associated morbidity and few complications. Most odontogenic cysts can be effectively removed by simple enucleation of the cystic lining and meticulous curettage of the bony cavity. However, when used alone, this technique is usually inadequate for tumors with true neoplastic potential and its use in entities such as ameloblastoma or keratocystic odontogenic tumor should be accompanied by adjuvant treatment, such as peripheral ostectomy, cryotherapy, or chemical fixation with Carnoy’s solution.
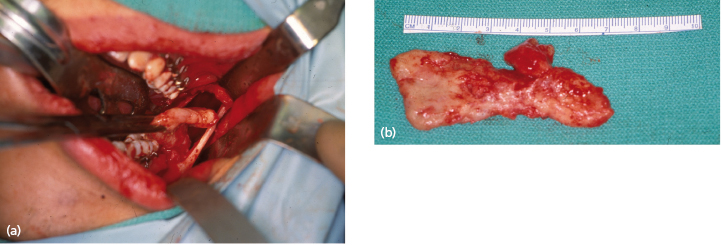
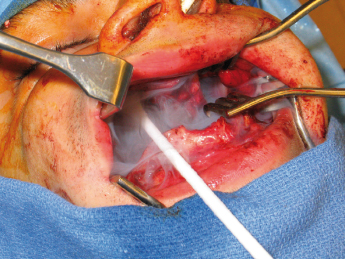
Wide exposure is necessary to allow complete access to the bony cavity (Fig. 21.2). Every effort must be made to thoroughly remove the cyst lining as well as epithelial remnants that may be present between the cyst wall and overlying mucosa. One of the difficulties encountered when attempting to remove these cysts lies in the nature of the thin cystic lining which, at times, is readily removed in toto, but more often comes out in multiple soft tissue fragments. The inferior alveolar nerve can be routinely spared and root canal therapy is almost never indicated. Most of the time, the mucosa can be closed primarily, without the need for bone grafts or packing material. Removal of a large tumor or cyst will occasionally weaken the remaining bony integrity, placing it at risk for pathologic fracture. This can be managed with 6 weeks of intermaxillary fixation and/or placement of a reconstruction plate. Enucleation and curettage is a reasonable method for the primary treatment of small, unilocular cysts that are typically not biopsied prior to definitive treatment.
Enucleation and Peripheral Ostectomy
Enucleation with peripheral ostectomy is an extension of the curettage technique described in Enucleation and curettage. It involves the use of a rotary instrument to remove bone adjacent to the cyst lining, theoretically facilitating removal of all residual epithelium and/or daughter cysts. Methylene blue staining is often used to aid in identification of appropriate bony margins. The technique can stand alone or include chemical or thermal fixation of the interior of the bony cavity. The advantage of peripheral ostectomy is that it provides an additional “margin” of bone removal during excision of the lesion and can potentially alleviate the need for adjunctive measures. The disadvantages are that it places other anatomic structures at risk of injury, i.e. teeth and the inferior alveolar nerve, and may further weaken the jaw structure. The use of peripheral ostectomy for treatment of a number of odontogenic tumors has merit in that it can facilitate a more “radical” surgery than curettage but is less morbid than resection.
Marsupialization
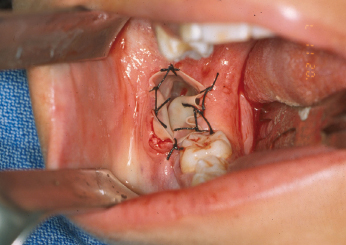
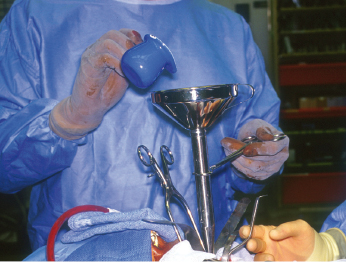
Marsupialization is a technique that is designed to initially decompress and shrink the cyst or tumor prior to definitive removal, generally by enucleation and curettage several months later. The primary advantage of this method is to minimize the surgical defect that is created by removal of the cystic lesion. This technique has been observed to cause inflammation and subsequent thickening of the cystic lining which facilitates its ultimate removal. Various inflammatory mediators may play a role in cyst volume reduction. Marsupialization has been shown to inhibit interleukin1-alpha expression within the lining of KCOTs, thereby halting epithelial cell proliferation and decreasing the size of the cystic tumor. The technique is performed by “de-roofing” the cyst and either repeatedly packing the cavity with gauze or by simply placing a tube, catheter or drain in order to facilitate gradual decompression and shrinkage of the defect (Fig. 21.3). The pack or drain is left in place for 2–3.
Intralesional Adjunctive Therapy: chemical Fixation
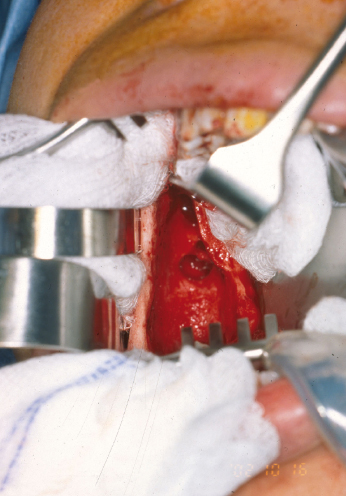
Remnants of the dental lamina may play a role in the etiology of some odontogenic tumors. The presence of epithelial islands within the mucosa overlying the cyst as well as the bony cavity has prompted the use of various surgical strategies to adjunctively treat the surrounding tissue in an effort to eradicate residual disease and minimize recurrence. The use of such adjuvant measures is perhaps best studied in the treatment of KCOTs. Stoelinga, Voorsmit, and colleagues advocate excision of the overlying mucosa and have popularized the use of Carnoy’s solution as a chemical tissue fixative for treatment of KCOTs. Carnoy’s solution is a mixture of absolute alcohol, chloroform, glacial acetic acid, and ferric chloride that penetrates bone to a predictable, time-dependent depth, without injuring the neurovascular structures, providing that it is not in contact with the inferior alveolar nerve for longer than two minutes. A 5-minute application will penetrate bone to a depth of over 1.5 mm. Because most residual cells and daughter cysts from locally recurrent lesions are adjacent to the main lesion, it is likely that fixation of vital bone need only extend for 2–3 mm beyond the enucleated lesion. Theoretically, enucleation of the cyst wall, excision of the overlying mucosa, and treatment of the surrounding tissue with Carnoy’s solution should sufficiently remove the cystic lesion along with any epithelial remnants remaining in the area. Application of the Carnoy’s solution can be either prior to enucleation or afterwards. Voorsmit’s original description of the technique called for treatment of the cyst before enucleation which causes a “tanning effect” of the lesion thereby facilitating complete removal. The author’s experience, however, is that treatment of the bony cavity after enucleation allows for easier removal of the cyst lining and better identification of soft tissue remnants.
Intralesional Adjunctive Therapy: cryotherapy
Cryosurgery with liquid nitrogen, a method of treating disease by the production of freezing temperatures in tissue, has been advocated as an adjunct to the surgical treatment of benign cysts and tumors of the jaws. Cryosurgery for osseous lesions produces cellular necrosis in bone while maintaining the inorganic osseous framework. Predictable cell lysis occurs at temperatures below −20°C and is caused by direct damage from intracellular and extracellular ice crystal formation, osmotic disturbances, and electrolyte imbalance. Similar to chemical fixation with Carnoy’s solution, cryosurgery allows for removal of the cyst or tumor by enucleation and curettage followed by treatment of the surrounding tissue. A single 1-minute freeze produces a depth of bone necrosis of 1–3 mm depending on the technique (Figs 21.4, 21.5 and 21.6). The techniques acceptable for oral cryosurgical use include the cryoprobe with water-soluble jelly and liquid nitrogen spray. The advantage of a cryoprobe with jelly is that it is possible to freeze irregular, gravity-dependent portions of the bony cavity. The disadvantage is that there is non-uniform freezing. The advantage of liquid nitrogen spray is potent, uniform freezing. The disadvantage is the potential for damage to surrounding tissues. Immediate bone grafting has been recommended for defects greater than 4 cm that are treated with cryotherapy. Bone grafting is thought to decrease complications such as wound dehiscence and pathologic fracture, while simultaneously providing greater residual bone height and density, thus facilitating implant placement.
Resection
The aggressive clinical behavior of some odontogenic and non-odontogenic jaw tumors is well recognized. Although rare, extension into vital anatomic regions occurs and can involve the skull base, infratemporal fossa, and/or orbit. Composite, segmental or marginal resection should be considered for some large primary or recurrent benign tumors, tumors involving the orbit, posterior maxilla, pteryopalatine fossa, skull base or infratemporal fossa, and malignant tumors.
Reconstruction
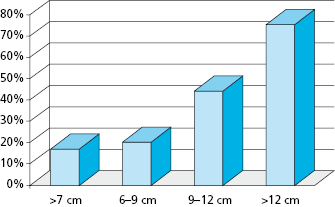
Ideally, benign or malignant ablation should be followed by immediate oromandibular or palatomaxillary reconstruction to re-establish function and achieve an acceptable cosmetic result. Immediate bony reconstruction of smaller defects can be performed by using non-vascularized autogenous bone grafts harvested from the ilium. Important factors that have been found to affect graft survival include the length of the mandibular defect, timing of the reconstruction (immediate versus delayed), pre- or postoperative radiation therapy, postoperative recipient site complications, malignant diagnosis, intraoral communication, estimated blood loss, the number of days on postoperative antibiotics, and the use of soft tissue flaps. In the primary setting, however, microvascular transfer of free osteocutaneous flaps from the fibula, iliac crest, radial forearm, or scapula seems to provide the most reliable means for reconstruction of composite or segmental defects with a success rate of around 95%. Length of the defect has been shown to be an important factor in bone graft survival for mandibular reconstruction. Foster et al. critically evaluated the use of non-vascularized grafts compared to microvascular composite free flaps in reconstruction of mandibular continuity defects and found that success was dependent in part on the length of the defect. Success rates in terms of osseous union using non-vascularized bone grafts decreased significantly in those patients with defects greater than 6 cm (Fig. 21.7). The overall success rate of non-vascularized bone grafts was approximately 69% while the overall success rate of vascularized bone flaps was 96%. Additionally, it took an average of 2.3 operations to complete the reconstruction for those patients with non-vascularized bone grafts as opposed to only 1.1 operations for those patients with vascularized bone flaps.
Surgical Treatment of Malignant Tumors of the Jaws
Malignant tumors typically require standard extra oral neck incisions in order to obtain proper access and facilitate reconstruction. A tracheostomy may be needed depending on the location and extent of the planned surgical resection. A neck dissection is generally required and, classically, it precedes surgical resection of the primary tumor. Classically, the tumor resection should be performed en bloc with the neck dissection and the tumor delivered along with the lymph node-bearing tissue.
Benign Tumors
Odontogenic Epithelium with Mature, Fibrous stroma without Odontogenic Ectomesenchyme
Ameloblastomas
The ameloblastoma is a true neoplasm of odontogenic epithelial origin. The etiology of ameloblastoma is not known with certainty but the cells of origin are thought to be ameloblasts. Excluding odontomas, ameloblastomas are the most common odontogenic neoplasm and account for approximately 10% of all tumors that arise in the mandible and maxilla. Larsson and Almeren described an incidence of 0.3 cases per million people per year in Sweden. On the other hand, studies from Africa suggest that ameloblastomas may represent more than 60% of all odontogenic tumors in that part of the world.
Presenting symptoms may include a slow-growing submucosal mass, loose teeth, malocclusion, paresthesia, and pain. As many as 35% of patients are completely asymptomatic and the lesions are discovered as incidental findings on routine dental radiographs. The median age at presentation is 35 years (range, 4–92 years) and there is no gender predilection. Eighty percent of ameloblastomas arise in the mandible, usually in the ramus region, and are often associated with molar teeth.
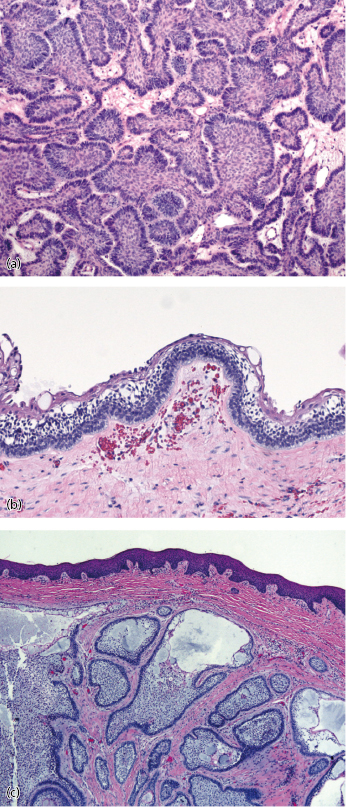
Ameloblastomas have a distinctive histological appearance manifested by columnar, basally staining cells arranged in a palisaded pattern along the basement membrane (Fig. 21.8). Six histopathologic subtypes are recognized, including follicular, acanthomatous, plexiform, basal cell, desmoplastic, and granular cell variants. Most tumors show a predominance of one pattern, but mixtures of different patterns are commonly observed. Granular cell ameloblastoma was at one time thought to be a more aggressive variant; however, this is no longer the case. Currently, most clinicians consider the histological subtypes to be of academic interest only and there is no convincing evidence suggesting a significant difference in biologic behavior. All ameloblastomas are benign but locally aggressive, will occasionally metastasize, and a few have malignant potential.
Solid/Multicystic Ameloblastomas
These normally present as locally aggressive tumors that demonstrate inherent neoplastic cellular proliferation, are associated with high recurrence rates when inadequately treated, and represent the most clinically significant odontogenic tumor based on potential morbidity and prevalence. If left untreated, these tumors can grow to extreme sizes (Fig. 21.9). Enucleation and curettage has been associated with a recurrence rate of 60–80%. Neoplastic cells have been shown to be present several millimeters from the radiographic margin of the tumor, which has led to the recommendation that resection be performed with care to maintain 1 cm tumor-free margins. It is clear, based on the current body of evidence, that enucleation and curettage alone is inadequate for definitive treatment of solid ameloblastoma. Resection is the gold standard and currently offers the most predictable recurrence-free treatment for solid ameloblastoma (Figs 21.10 and 21.11). For many tumors, however, resection with 1 cm tumor-free margins will commit the surgeon and patient to a segmental mandibulectomy or maxillectomy, often with sacrifice of the inferior alveolar nerve or creation of an oral–antral communication, and will necessitate primary or secondary reconstruction of continuity defects of the mandible or maxillectomy defects. Techniques have been described for segmental mandibular resection with inferior alveolar nerve preservation; however, this technique runs the theoretical risk of inviting perineural recurrence. Since ameloblastoma is a benign disease, many surgeons are reluctant to perform radical surgery on these patients. This understandable reserve has led to a number of other techniques including enucleation with peripheral ostectomy and/or adjuvant treatment with liquid nitrogen cryotherapy.
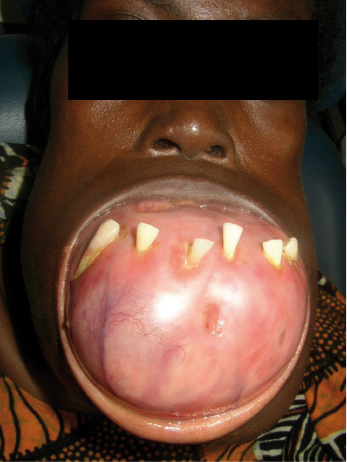
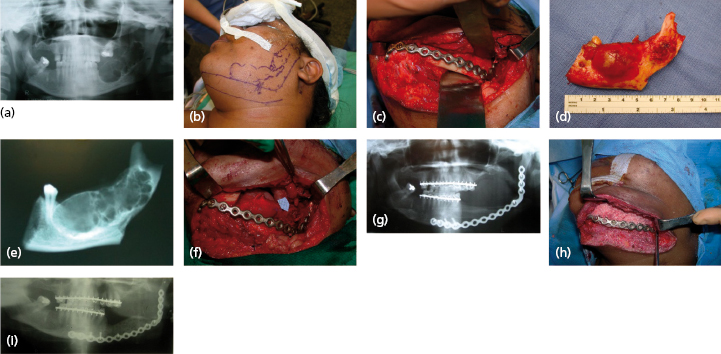
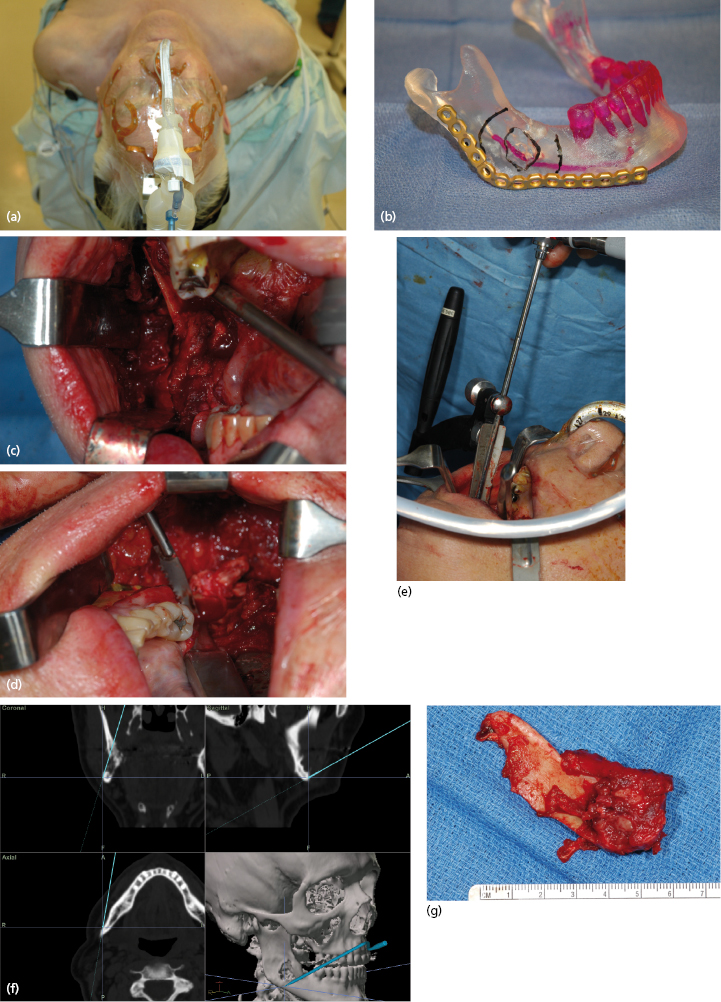
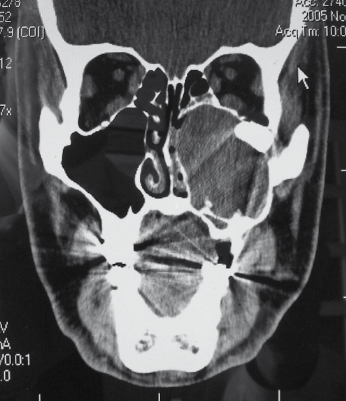
Although most ameloblastomas occur in the mandible, maxillary ameloblastomas are often more problematic than mandibular tumors (Fig. 21.12). Although histologically identical to their mandibular counterpart, the surrounding anatomy is more complex. Involvement of the maxillary sinus and nasal cavity commonly occurs and infiltration into the orbit, pterygomaxillary space, and skull base is distinctly possible in some patients with recurrent or uncontrolled disease. Even simple enucleation and peripheral ostectomy may result in oral–antral communication and thus require reconstruction. If it is possible to perform excision with peripheral ostectomy and treatment with liquid nitrogen therapy without creating an oral–antral/oral–nasal defect, then this is the preferred treatment for most small to medium-sized lesions. However, because of the reconstructive issues, the general approach for larger tumors is to perform resection and immediate reconstruction using either local or regional rotational flaps, or alternatively microvascular free flaps or an obturator.
Unicystic Ameloblastoma
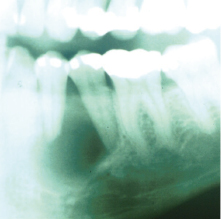
Unicystic ameloblastoms represent a subgroup seen in approximately 6% of ameloblastomas and were first described in 1977. They tend to occur in a younger population (mean age 22.1 years) and a high percentage of these lesions are associated with an impacted tooth. They will often present as a well circumscribed, unilocular periapical radiolucency (Fig. 21.13). Histologically, the unicystic ameloblastoma shows a cystic architecture with the typical ameloblastic changes confined to the cyst-lining epithelium (Fig. 21.8b). While most pathologists accept the concept of unicystic ameloblastoma as a separate entity rather than as a precursor to solid ameloblastoma, much controversy exists over the terminology and how it should be classified.
One of the contentious issues regarding unicystic ameloblastoma is related to proliferation of the ameloblastic epithelium into the lumen of the cystic cavity or into the adjacent bone. Extension of the ameloblastic epithelium into the lumen of the cystic cavity is termed intraluminal, or mural proliferation. There seems to be general agreement that as long as the ameloblastic characteristics are confined to the lining epithelial layers or if it remains intraluminal, conservative therapy such as enucleation and curettage is sufficient treatment. On the other hand, transmural involvement of the epithelium and bony invasion is defined by some pathologists as solid or “invasive” ameloblastoma, a term more descriptive of its aggressive biologic behavior.
It is clear that some unicystic ameloblastomas have a better prognosis and less chance for recurrence than do multicystic or solid variants when treated with enucleation and curettage. It is also clear that the unicystic ameloblastoma is considerably more aggressive than a simple dentigerous cyst, with which it is included in a differential diagnosis.
The clinical problem rests in the fact that the diagnosis is often made retrospectively once the patient has undergone definitive treatment for a presumptive odontogenic cyst. The issue is complicated by the fact that to definitively establish a lesion as a unicystic ameloblastoma, complete microscopic evaluation of the bony margin surrounding the lesion must be performed, therefore necessitating en bloc resection of the osteolytic lesion. Clearly this is impractical and would represent gross overtreatment of the vast majority of lesions. Since incisional biopsy can never provide a definitive diagnosis, it is doubtful that the issue will be settled in the near future. The surgeon should consider all of the clinical, radiographic, and patient-/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


