Chapter 14
Implantology
Evidence-based oral implant therapy was presented to the dental community in the early 1980s. Foreign materials have, however, long been used to replace missing teeth. For example, subperiosteal and blade implants had been used for some decades when pure titanium screw-shaped implants were introduced by the experimental and clinical work of P-I Brånemark. Today, the use of osseointegrated oral implants is considered to be standard procedure for the treatment of total and partial edentulism. Long-term clinical data on modern implant systems on the market today have, in general, shown high survival rates and minimal marginal bone loss around the implants. However, critical reviews of the scientific literature have concluded that some, often low-price systems, are not yet sufficiently documented in long-term clinical trials.
One prerequisite for successful anchorage and long-term function of titanium implants is that a sufficient volume of healthy bone is available in order to house adequate numbers and sizes of implants. The density of the bone is also a determinant of implant success, since failure is more common in bone with low mineral content.
Biological Principles Behind Osseointegration
Implant Stability and Loading Considerations
In the early days of implant dentistry, the use of osseointegrated implants always included a healing period of typically 3–6 months from placement to prosthetic treatment. The formation of direct bone–implant contacts was considered a prerequisite before loading could be commenced (Fig. 14.1). Today, immediate/early loading of dental implants is a clinical reality and numerous clinical studies have demonstrated as good results as those previously reported for two-stage implants. This has changed the understanding of what factors are important for a successful clinical outcome. In the early days, focus was mainly paid to the osseointegration process per se, whilst today a general stability concept is discussed where bone healing is one of several important factors.
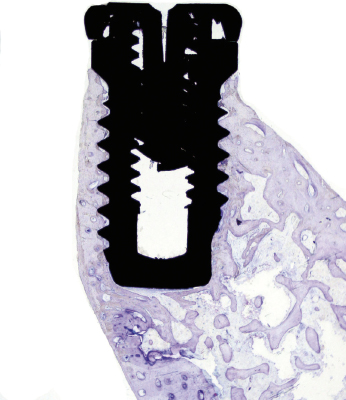
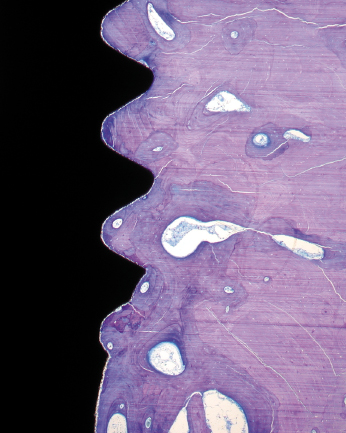
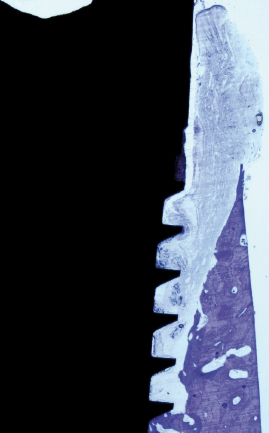
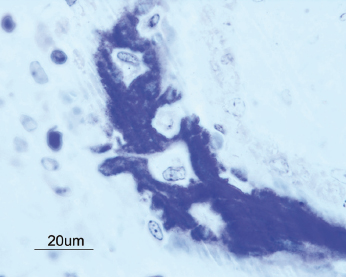
The clinical manifestation of a successful dental implant is the absence of mobility. Thus, achievement and maintenance of implant stability are prerequisites for a successful clinical outcome with dental implants. The main determinants of implant stability are: (1) the mechanical properties of the bone tissue at the implant site and (2) how well the implant is engaged with that bone tissue. The first factor is determined by the composition of the bone at the implant site and is influenced by stage of healing, since soft trabecular bone seems to be transformed to dense cortical bone near the implant surface. The second factor is influenced by the surgical technique, the design of the implant, and the osseointegration process. Successful healing results in bone formation that reinforces the interface zone and forms bridges and a direct contact between the implant surface and the surrounding bone (Fig. 14.2). Unsuccessful healing results in formation of an interface fibrous scar tissue (Fig. 14.3), which can be caused by infection or mobility of the implant after installation.
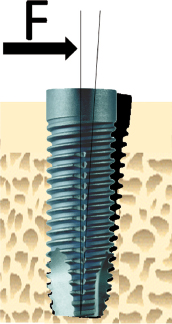
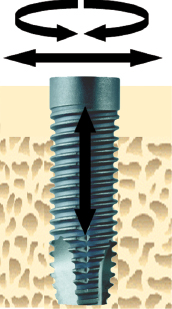
However, a clinically stable and successful implant also shows a certain degree of mobility on the microscale when a load is applied. For instance, if applying a lateral load (bending) to an implant in bone, the implant will be displaced but will return to its original position as soon as the load is removed (Fig. 14.4). Thus, a stable implant can display different degrees of stability, i.e. different degrees of displacement or resistance to load, depending on factors relating to the bone, the surgical technique, and the implant design. During clinical function, loading will be applied in axial, lateral, and rotational directions (Fig. 14.5). Furthermore, axial loads can be in push-in or pull-out directions. Lateral loads can principally be applied from any direction 360° around the implant. Rotational loading can either be clockwise or anticlockwise. Thus, the outcome of implant stability analysis depends greatly on the type of test used and in which direction the load is applied. In essence, stability measurements in bending give information about the stiffness of the implant–bone system, while application of shear forces, with for instance a reverse torque test, measures the strength of the interface. This means that a newly placed implant can show a high degree of lateral stability but is easily removed when applying reverse torque since bone has not been formed and interlocked with the implant surface. With time, bone formation will lead to an increased interlocking with the implant surface and an increased strength of the interface, whilst the lateral stability may be unaffected. Since most implants will be connected with a framework, reverse torque tests are probably less relevant than measurements of lateral stability.
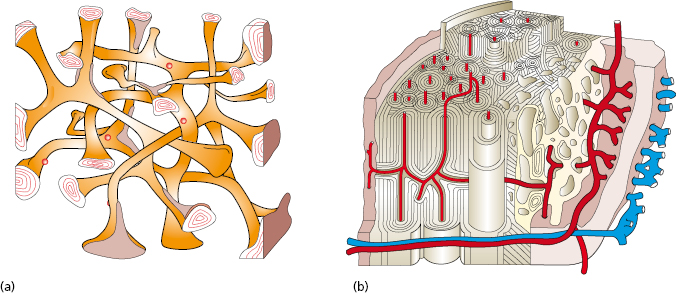
Primary Stability
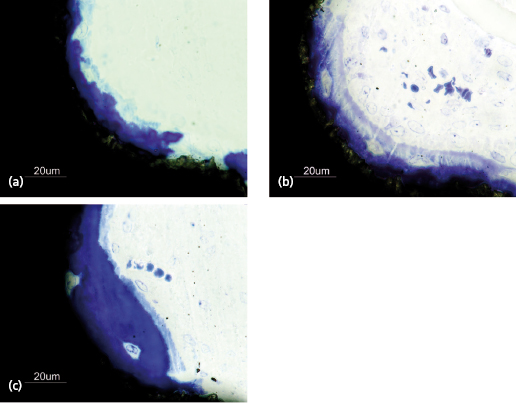
Implant stability is the result of contact between the implant surface and surrounding bone tissue. The degree of primary stability after installation depends on factors related to the bone, surgical technique, and implant design. The biomechanical properties of bone are determined by the ratio of cortical and trabecular bone at an implant site. Cortical bone is built up of densely packed mineralized lamellae, whilst trabecular bone is porous in structure and contains more soft tissue components than mineralized tissue (Fig. 14.6). For this reason cortical bone is 10–20 times stiffer than trabecular bone and provides a better support for an implant. The surgical technique can influence implant stability depending on the choice of drill diameters, depth of preparation, and whether pretapping is used or not. The implant itself also has impact on stability depending on its geometrical features. In a human cadaver study, it was demonstrated that bone density and implant design are important factors that influence primary stability as measured by resonance frequency analysis. They also found that 1° tapering of an implant dramatically increased the primary stability in poor bone. In continuing work, it was shown that primary stability also is influenced by drill diameter and whether pretapping is used or not. In essence, thinner drill diameters, omitting pretapping, and the use of a tapered implant will result in higher primary stability.
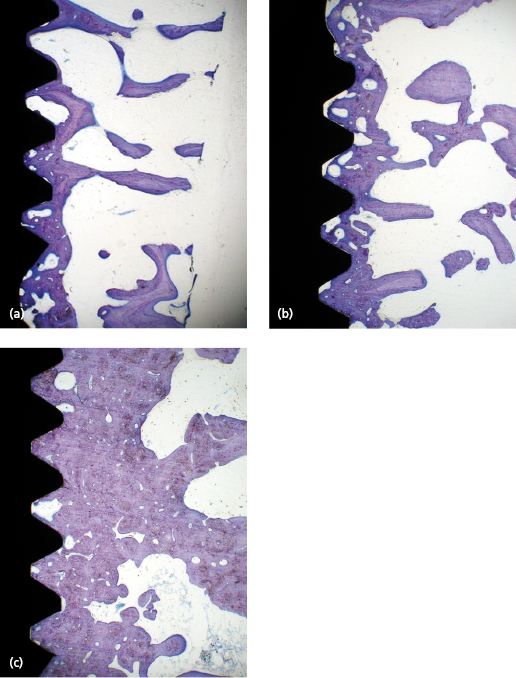
Secondary Stability
After implant placement the bone tissue will respond to the surgical trauma, which with time results in: (1) a change of the cortical/trabecular bone ratio and (2) an increasing degree of bone–implant contact. The time needed for completion of bone formation and remodeling is in the range of 12–18 months. The impact of the degree of bone–implant contact for secondary implant stability is not known in detail, although it is generally anticipated that more bone contact means better implant stability. However, the change of the trabecular network into a more cortical bone in relation to the implant surface is probably more important. This means that the biomechanical properties of trabecular bone improve with time which leads to greater stability of the implant (Fig. 14.7). Cortical bone already has favorable properties from an implant stability point of view. Therefore, histological changes of cortical bone will not necessarily lead to an increase in secondary stability (Fig. 14.8).
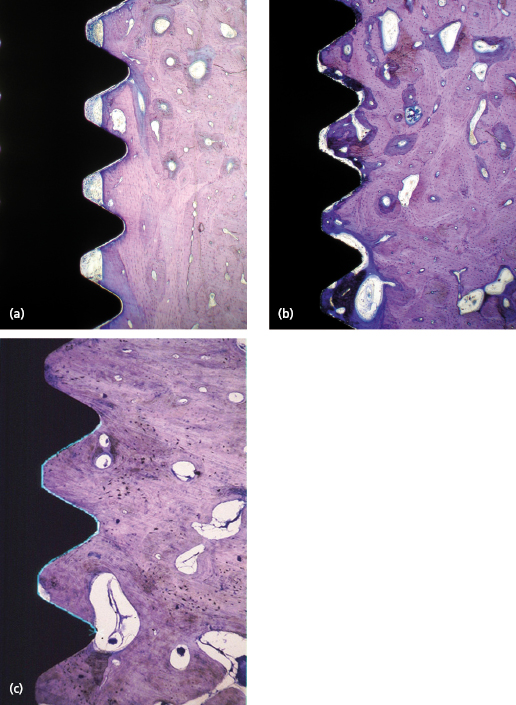
The time needed to achieve sufficient implant stability greatly depends on the density of the bone and thereby the primary stability, i.e. an implant with low primary stability needs a long period of healing whilst an implant with high stability needs only short or no healing. The host’s ability to maintain and to increase the primary implant stability is also determined by the healing and remodeling capacity, which in turn is influenced by endogenous and exogenous factors such as health, the use of drugs, smoking, irradiation, etc.
Maintenance of Stability
When a crown/bridge or an overdenture has been connected to the implants, the conveyed loads and stresses will have an influence on the bone physiology. In the early phase, the implant–bone system will be loaded while bone formation and remodeling induced by the surgical placement are still ongoing. It is reasonable to suggest that the bone tissue is more sensitive at this stage than after completed healing as most failures occur during the first year of loading. If the loads are excessive there is an obvious risk that this may lead to resorption, decrease of stability, and eventual loss of the implant. If the loads are within physiological limits it is probable that loading may stimulate further remodeling and adaptation of the bone to the present load situation. Overload has to be looked upon as a relative parameter since this describes an imbalance between loads and the degree of implant stability. In other words, for a given load the overload threshold is lower for an implant with low stability than for one with high stability.
Bone Tissue Responses to Implants
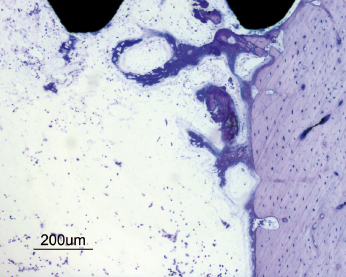
The bone healing process around a dental implant resembles that of normal bone healing. The surgical trauma created during the insertion of an implant initiates an immediate and preprogrammed healing response at the bone–implant interface. This process involves the formation of a blood clot containing erythrocytes and biologically potent cells such as leukocytes and thrombocytes. Cytokines and factors from these cells, the coagulation process, and injured tissues act as chemotactic stimuli on leukocytes and other cell types. The fibrin network within the blood clot provides an important scaffold for migrating cells involved in the formation of new tissues, such as vessels, extracellular matrix, and bone. Mesenchymal cells in the granulation tissue will differentiate to preosteoblasts and subsequently osteoblasts and start to produce immature woven bone (Fig. 14.9). If a certain surface topography is present, new bone formation will occur directly at the implant surface (Fig. 14.10). Bone formation is also seen at the pre-existing bone surfaces facing the implant, probably as a result of activation of so-called resting cells and by populations of newly differentiated stem cells (Fig. 14.11). With time the new bone from adjacent bone surfaces will reach the implant surface and create bone–implant contacts and fuse with the bone initially formed in the granulation tissue near or at the implant surface. A rapid increase of new bone area and degree of bone–implant contact is seen during the early healing period (Fig. 14.12). Bone regeneration occurs in two stages and this is also the case around dental implants. The immature woven bone is replaced by mature lamellar bone through a remodeling process. This is done by bone metabolizing units (BMUs), which contain osteoclasts, which resorb the woven bone, followed by a seam of osteoblasts, which lay down new layers of bone (Fig. 14.13). It is anticipated that the early bone formation takes about 3–4 months, whilst the remodeling process of the repair may take another 9–12 months in humans. However, physiological remodeling is a continuous process since it is part of the calcium metabolism system (Fig. 14.14).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


