Chapter 13
Root filling techniques
Introduction
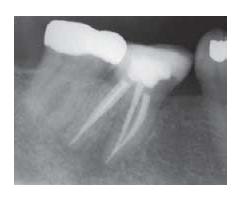
Filling the instrumented root canal is the final step in the completion of an endodontic treatment. Regardless of whether the treatment was undertaken to remove a vital pulp (pulpectomy), a necrotic and/or infected pulp (root canal therapy) or a previous root canal filling (retreatment), the prime objective of the root filling is to prevent microbial organisms from entering, growing and multiplying in the empty space that resulted from the instrumentation procedure (Fig. 13.1). Root filling also serves as a wound dressing against which healthy periapical tissue can be laid down.
Specific objectives
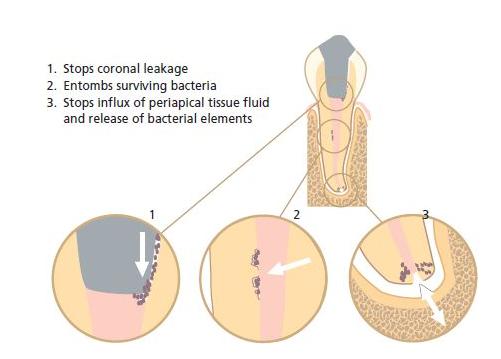
After pulpectomy a wound surface remains that will not heal with epithelium as wounds do in other body sites. Such a wound surface is therefore constantly vulnerable to infection. Wound infection may be induced inadvertently in conjunction with the treatment procedure, e.g. from improper rubber dam isolation or by bringing chips of carious dentin to the apical region of the root canal. It may also develop from entry of bacterial organisms from the oral environment after completion of the filling. The latter is known as coronal leakage and occurs along incompletely sealed canal spaces. Therefore, a hermetic and permanent seal of the wound surface is essential to allow proper healing after pulpectomy and to prevent bacterial elements from later accessing the periapical tissue if, for any reason, the coronal restoration breaks down. Core concept 13.1 summarizes the overall functions of a root filling.
Fig. 13.1 Radiograph depicting optimal root canal fillings of a lower molar. (Courtesy of Dr A. Braun.)
In the treatment of a tooth with an infected, non-vital pulp (i.e. root canal therapy), instrumentation and irrigation with a disinfecting solution will not always eliminate the microbial organisms. If such a root canal is left unfilled or improperly filled, residual organisms may continue to grow and multiply (Chapter 9). It needs to be recognized that microbial organisms require both space and nutrition for growth, therefore in root canal therapy the root filling has two additional objectives:
Usually it is sufficient to block the portal of exit to the periapical tissue. However, lateral or accessory canals may also allow egress of bacterial elements to the periodontium. Therefore it is essential that the entire length of the instrumented canal becomes completely filled. If properly done this means that all portals of exit to the periodontal tissue will be sealed. The quality of root fillings in general is assessed from this aspect (see further below).
Selecting a root canal filling material
A variety of factors determines the choice of a root canal filling material. Although a primary requirement is to allow a complete fill of the instrumented root canal(s), it should also be biologically compatible because it will often be in direct contact with vital tissue. In other words, beside a variety of technical and physical demands, a root filling material should also satisfy the requirements that are requested from implant materials (Chapter 12). The most critical technical and physical requirements of a root filling material are:
- Ability to adapt to the shape of the canal. After cleaning and shaping, root canals may still harbor various irregularities that can allow space for bacterial growth. It has been shown that in many cases it is impossible to create a round and smooth root canal without removing so much of the inner root canal wall that the root structure is critically weakened. Therefore, to provide a seal, a root canal filling material should be able to fill these irregularities.
- Length control. A root canal filling material should allow a technique that keeps the entire material within the canal space. Extrusion of material to the periapical tissue compartment is undesirable because it may cause both cytotoxic and neurotoxic effects (7, 29, 40). It may also produce a foreign body reaction (40). Furthermore, results of clinical outcome studies indicate that extrusion and overextension of root filling material negatively influence the healing of the periapical tissue (12, 32).
- Safety. The material and the technique used for its application should be safe for the patient. The demand for biocompatibility has already been stated (see Chapter 12), but the technique should also not pose risks for root fracture, require overzealous instrumentation or cause damage to the periodontal ligament by, for example, detrimental temperature increases or extrusion of material.
- Insoluble. Because of the risk for coronal leakage and the fact that root filling material may be exposed to percolation of tissue fluid at the apical foramen, it is important that it is not affected by moisture. Therefore, after setting, a root filling material should be insoluble in both saliva and tissue fluid.
- Removable. A root filling may not be performed perfectly at the first attempt or may turn out to be defective at a follow-up. The outcome of a treatment may also be such that one suspects ongoing root canal infection. In these instances, retreatment and refilling of the root canal may be necessary (Chapter 20). The material used should, therefore, be removable by simple means without involving the risk of damaging the root structure or the apical tissue.
- Radiopaque. In order to judge whether the root canal has been adequately filled, a most important requirement is that the root filling is discernible in a radiograph, i.e. it should be radiopaque.
Hardly any root filling material so far developed has been able to satisfy all these demands. Yet a formulation based on gutta-percha as one of the principal ingredients has stood the test of time and has been widely used since the end of the 19th century. Although new core materials are under exploration it is still the material of choice in most countries. By pressure or by softening with heat or organic solvent, gutta-percha is suitable for application in instrumented root canals using a variety of techniques (see further below). Combined with a sealing agent, the material can be adapted to the shape of root canals and serve as a reasonably insoluble and non-porous core of filling. At the same time, it is fairly easy to remove if necessary. Gutta-percha-based formulations also satisfy biological demands (Chapter 12). Because of the universal use of gutta-percha in endodontics, techniques that are based on this material will mainly be considered in this chapter.
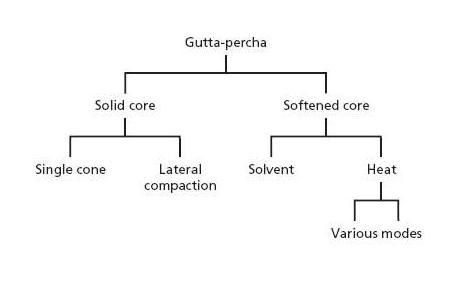
Fig. 13.2 Outline of techniques to fill root canals with gutta-percha.
Root filling techniques for gutta-percha
There are various methods for delivering and packing gutta-percha in root canals and they can be divided into solid core and softened core techniques (Fig. 13.2). Solid core techniques imply that unsoftened gutta-percha cones are fitted to the instrumented canal(s) (Fig. 13.3a) and cemented to the canal walls with a root canal sealer. Techniques exist whereby either a single cone or multiple cones are placed in the root canal space (Fig. 13.4). In softened core techniques gutta-percha is plasticized either prior to or after insertion in the root canal by solvent or heat. These techniques also often make use of a sealing agent to supplement the filling.
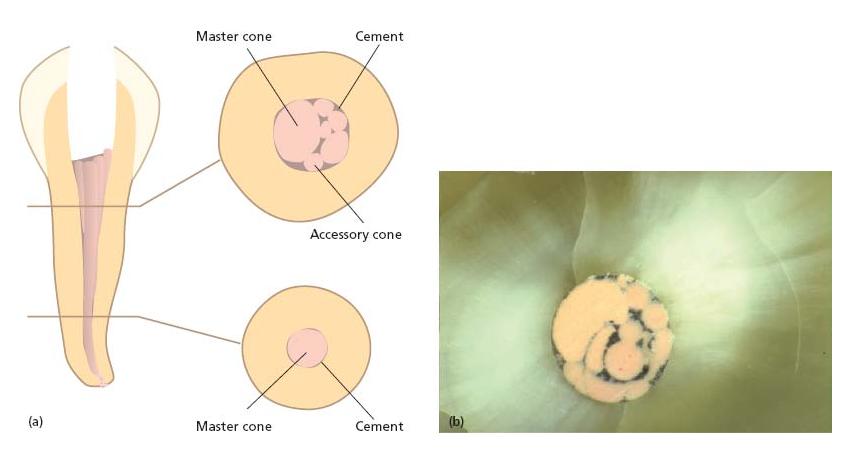
Fig. 13.3 Outline of the lateral compaction technique. (a) Master cone fit. (b) Lateral compaction with spreader following addition of one accessory cone. (c) Continued lateral compaction. (d) Further addition of accessory cones. Root filling is complete when it is not possible to place another accessory cone further than 2 mm into the root canal.
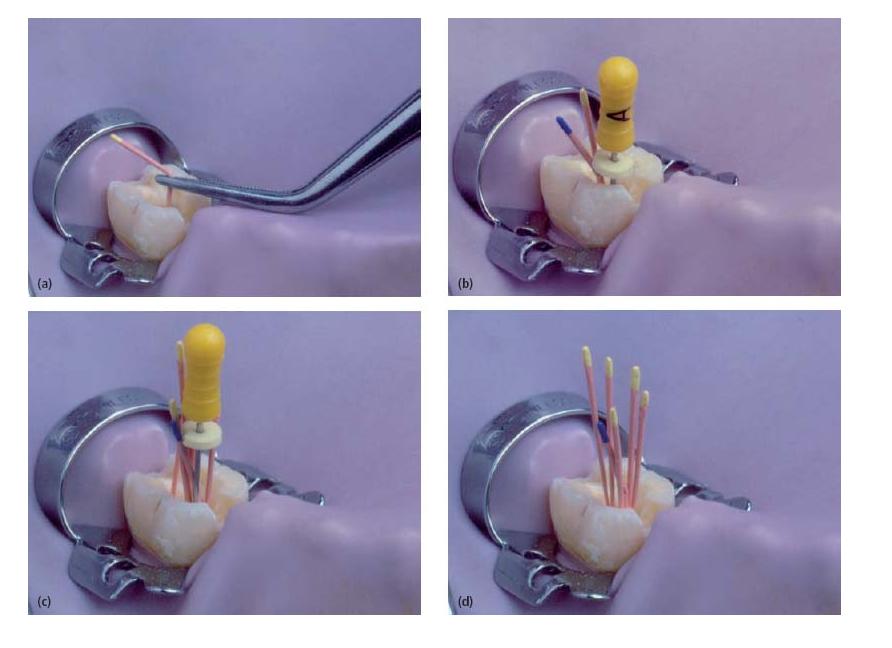
Fig. 13.4 (a) Sketch showing a cross-sectional cut through a root canal filled with a master cone and multiple accessory cones. (b) The cross-sectional cut shows a true filling where the sealer (black material) unites the cones and fills out space laterally to the root canal wall.
Gutta-percha cones
For solid core techniques, gutta-percha cones of different lengths, sizes and shapes may be obtained. In general, cones are round and have a tapered form so they gradually increase in size from the tip. So-called “standardized” cones were designed to match the size and taper of the root canal instruments used to shape the canal at its apical end. In early days these cones had a rather small taper of 2%, corresponding to the ISO standard of root canal instruments. Nowadays there are cones standardized to fit canals prepared with differently tapered instruments. Hence, there are cones with 4% and 6% tapers. “Conventional” cones are also available; these are not standardized and are classified as fine, medium and large.
Root canal sealers
Unsoftened gutta-percha does not adhere to dentin and softened gutta-percha may shrink after cooling as a result of being heated or from evaporation of the solvent used, thus leaving gaps between the material and the root canal walls (50). Naturally such defects may allow either coronal or apical leakage, or both, to cause or maintain apical periodontitis. It is, therefore, considered necessary to use a cement or sealer that forms a tight connection between the gutta-percha and the root dentin. In general, it is believed that this layer should be as thin as possible because, upon setting, sealers may shrink and dissolve in a moist environment (20).
This has led to the development of gutta-percha techniques that aim to create a filling consisting of a well-adapted mass of gutta-percha with a thin layer of root canal sealer between the gutta-percha and the root dentin. In this respect, consideration is similar to that with cast restorations, where well-fitting margins are created to leave as little cement as possible between the metal and the tooth structure.
As a general trait, but to a varying extent, root canal sealers in the initial setting phase are cytotoxic and bacteriotoxic. Although most sealers become substantially less bioactive thereafter (29, 45), as little contact as possible with the apical pulp tissue or periapical tissue is desirable. In particular overfilling of sealer material should be avoided. There are several reasons for this view:
Currently used root canal sealers may be divided into four groups:
- zinc oxide–eugenol based;
- resin based;
- dentin-adhesive materials;
- materials to which medicaments have been added.
The benefits, uses and problems of each are discussed below and summarized in Core concept 13.2. For a more detailed description of these materials, the reader is referred to Chapter 12.
Zinc oxide–eugenol-based sealer
Once zinc oxide–eugenol materials set, they form a weak porous product that is decomposed in tissue fluids over time (25, 44, 46). Nonetheless, these materials are regarded to be clinically satisfactory (28). Practically all zinc oxide–eugenol sealers are cytotoxic and the response may be more long lasting compared to that of most other sealers owing to release of free eugenol upon gradual hydrolysis (29). Potential for sensitization exists (13, 15). Zinc oxide–eugenol cements are commercially available as Hermetic, Tubliseal, Procosol, Roth’s sealer and Kerr pulp canal sealers, and form the basis of many medicament-containing sealers.
- Reasonable seal
- Dissolve in fluids
- Long-lasting cytotoxicity
- Sensibilization
- Good seal
- Initial cytoxicity
- Once set, biocompatible
- Allergenic
- Moderate seal
- Initial cytotoxicity
- Shrinkage
- Plasticize gutta-percha
- Good seal
- Set very quickly
- Good biocompatibility
- Difficult to remove
- Zinc oxide–eugenol based
- Severe long-lasting cytotoxicity
- Sensibilization
- Release calcium hydroxide, which may result in disintegration
- Once set and integrity is maintained, no calcium hydroxide leaches out and no effect can be expected
- Initial antibacterial effect
- Risk of dissolution over time
Resin-based sealers
Well-known resin-based materials are AH26, AHPlus and Diaket. Both AH26 and AHPlus consist of an expoxy resin. They are thin fluid materials that set slowly. The long setting time may sometimes be an advantage because it gives sufficient time to correct deficiencies in the root canal filling that were noticed at the postoperative radiographic check. Diaket is a mixture of vinylpolymerizates that sets rapidly within minutes, which makes it less suitable in techniques that require some working time.
These resin-based sealers elicit an initial severe inflammatory reaction that subsides in subsequent weeks and thereafter becomes well tolerated by the periapical tissues (28, 29). However, the sealer AH26 has been shown to have both a strong allergenic and a mutagenic potential (15, 26) and contact allergy to this material has been reported (16).
Sealer based on silicon, e.g. RSA Roekoseal, GuttaFlow, is a recent development. The advantage of this material is volume stability and biocompatibility. One product, RSA Roekoseal, a polydimethylsiloxane, appeared to seal straight canals well in vitro with a single gutta-percha cone even after a long-term exposure to water (51). Another product, GuttaFlow, has the same composition as RoekoSeal except for additions of gutta-percha (<30 μm) and nanosilver particles for radiographic contrast. It has been introduced to the market as the first non-heated, flowable gutta-percha that, unlike heated gutta-percha, does not shrink. A recent evaluation of its sealing capacity seemed not very favorable (30) but its toxicity appeared low (6).
Dentin-adhesive materials
Adhesive cements have been tested as root canal filling material in an attempt to improve the sealing quality of sealers. Cyanoacrylate, calcium phosphate, polycarboxylate and glass ionomer cements have all been explored. Of these, only glass ionomer cements have been widely marketed (Ketac-Endo, Endion). Although appearing favorable in biocompatibility tests and in long-term clinical follow-up studies, the materials have never gained great popularity. Likely reasons are short setting time (20) and difficulty of removal for retreatment.
Materials to which medicaments have been added
These materials may be divided into two groups:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses