Chapter 12
Root canal filling materials
Introduction
Purpose
In addition to this traditional concept of the purpose of a root canal filling material, it has recently been put forward that a root canal filling material should be able to actively stimulate tissue regeneration, especially after a sometimes aggressive treatment procedure or after apical pathosis. Relevant materials may be osteoconduc-tive (serving as a scaffold for the ingrowth of precursor osteoblasts) or osteoinductive (inducing new bone formation by differentiation of pluripotent local connective tissue cells into bone-forming cells).
Classification
Root canal filling materials may be divided into three types:
- cones;
- sealers;
- combinations of the two.
Cones are prefabricated root canal filling materials of a given size and shape (taper). Sealers are pastes and cements that are mixed and hardened by a chemical setting reaction after a given amount of time. Setting time varies between the various preparations, from minutes up to days. Owing to several reasons outlined later, combinations of cones and sealers are currently recommended. Thermoplastic materials prepared from gutta-percha are gaining increasing interest and are heated for better adaptation to the root canal wall. They may also be melted and injected into the root canal in a liquid state and then hardened by cooling. Again, it is usually recommended to use these materials together with a sealer.
Limitations
As will be shown in this chapter, all materials recommended for root canal filling have advantages and disadvantages and there is no material/method yet available that fulfills all the requirements. Therefore clinicians are well advised to observe new developments and the relevant scientific literature carefully. It also should be kept in mind that clinical properties of root canal filling materials depend substantially upon the treatment technique: e.g. the amount of sealer used may determine the tissue reaction and the amount of leakage of some materials, due to factors such as shrinkage during setting, the formation of pores and enhanced solubility (45). Therefore, the selection and the use of a root canal filling material must be part of a whole treatment concept. Finally, there is no magic material by which the tedious work of correct diagnosis and chemomechanical preparation of the root canal system can be avoided.
Selection
Root canal filling materials should be selected on the basis of a critical evaluation of the presented evidence (preferably reports in scientific journals) in relation to the requirements, which will be mentioned below. Sometimes, however, contradicting results are reported for the same material. This may be due to the particular circumstances of both the test method used and the preparation of the specimens (tested freshly after mixing or in a set state). Thus, the clinician should ask for a set of tests preferably performed in a comparative (i.e. controlled) way, testing the new product against one or more currently accepted preparations. Selection of a suitable root canal filling material is a challenge for the clinician regarding both his or her level of updated information and his or her ability to critically assess presented information.
Requirements
Root canal filling materials may be considered as implants and thus should fulfill the requirements of such materials concerning technical, biological and handling properties (Core concept 12.1).
Technical properties
Technical properties are mainly related to sealing aspects, taking into account that the success of a root canal filling significantly depends upon the prevention of infection/ reinfection of the apical and lateral periodontal ligament and the adjacent bone. In cases of material extrusion beyond the apex, which is associated with elevated rates of clinical failure (79), resorption of the material would be desirable. However, this is in contradiction to the required insolubility. Therefore utmost care must be taken to avoid overfilling.
- No shrinkage
- No solubility in tissue fluids, undisturbed setting in the presence of moisture
- Good adhesion/adaptation to dentin or combining materials (cones, sealers)
- No pores and water absorption
- No tooth discoloration
- No general health problems or allergies for patients and dental personnel
- No irritation of local tissues
- Sterile
- Antimicrobial – no enhanced bacterial growth
- Stimulation of the periapical healing process
- Radiopaque: ISO 6876 (76) requires >3 mm aluminum (dentin has 0.6-0.7) (radiopacity of dental materials is measured as mm aluminum equivalent)
- Setting in an adequate time, allowing sufficient time for obturation and radiographic control
- Easy to apply and easy to remove (e.g. for post placement or revision) using solvents, heat or mechanical instrumentation
Biological properties
Biological properties are related to preventing systemic and local tissue irritation for both the patient and the dental personnel and providing the potential for regeneration of the apical tissues. The risk (frequency and severity of adverse effects) for general health impairment as a consequence of the use of root canal filling materials is generally low. Single cases of allergic reactions of patients and medical personnel have been reported. Local effects are more dramatic, especially in the context of overfilling beyond the apex and eventually into the mandibular canal (see below).
There are some inherent contradictions between the requirements for a root canal filling material that have to be weighed against each other, e.g. antibacterial properties versus local toxicity. Bacteria in the root canal should be removed by chemomechanical debridement. However, the complex anatomy of the root canal system (e.g. lateral canals) makes debridement difficult, especially in the apical delta region (see Chapter 11). Furthermore, bacteria have been shown to invade dentinal tubules and thus they may not be removed totally by chemomechanical debridement. Thorough cleaning, shaping and irrigation with disinfectants may not, therefore, result in a completely sterile root canal system. Owing to the fact that leakage cannot be totally prevented by any material/method available today, percolation of nutrient-rich fluid followed by bacterial regrowth may occur. Antimicrobial activity of root canal sealers may compensate for these imperfections, although this is not supported by direct scientific evidence.
It should be recognized that sealers with high antimicrobial activity, especially formaldehyde-releasing ZnOE (zinc oxide–eugenol), are also toxic to cells and tissues. Furthermore, sealers that release antimicrobial substances may disintegrate at the same time. Therefore, antibacterial properties of a root canal filling material based on the release of antibacterial substances from the sealer should not compromise its physical properties (such as stability and sealability) or biological properties. Some materials (e.g. epoxy resin sealers) are only antimicrobi-ally active during the setting period, which is an interesting approach. For a short period residual bacteria may be killed (toxicity is accepted); in the long run, the material is not toxic, leaving time for the surrounding tissues to heal (Fig. 12.1).
Handling properties
Handling properties will facilitate the actual use of the material and the control of the technique/treatment result. The length of the root canal filling is of utmost importance for clinical success and a sufficient radio pacity is needed for radiographic control. Setting conditions must be adjusted to the particular clinical situation the root canal filling techniques are aimed at and relevant requirements may be different for regular root canal fillings (slow setting allowing for condensation and eventual correction after radiographic control) and retrograde fillings (fast setting for better moisture control during the operation).
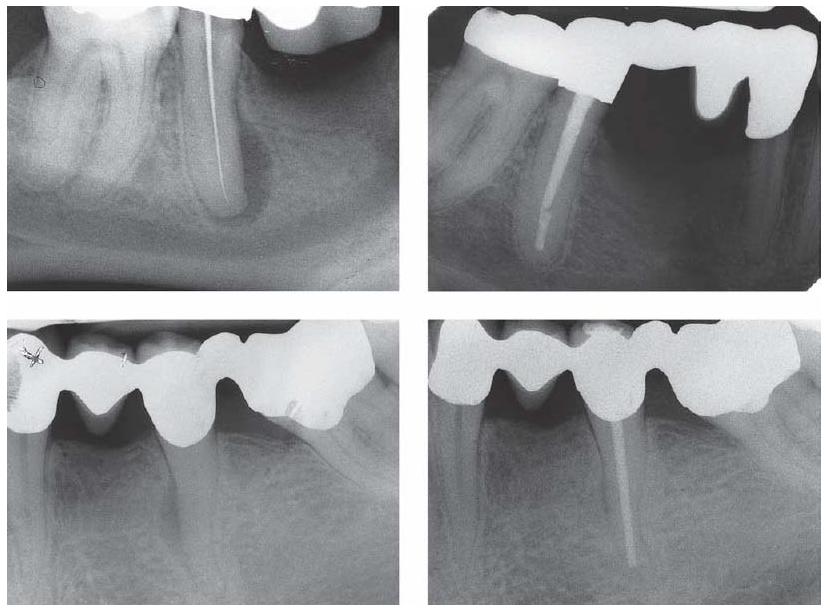
Fig. 12.1 Lateral and apical regeneration of an osteolytic process in two cases after shaping and cleaning the root canal and filling with cones and a sealer of temporary toxicity (gutta-percha with an epoxy resin sealer).
The ideal root canal filling material has not been developed yet. Compromises have to be made between the different requirements in relation to the individual clinical situation. New formulations, however, should be checked critically against the list of requirements (Core concept 12.1).
Biocompatibility
An acceptable level of biocompatibility is an essential requirement for a suitable root canal filling material. According to EU regulations (Medical Device Directive 93/42 EEC) valid within the EU and in Switzerland, Iceland, Liechtenstein and Norway, root canal filling materials have to successfully pass a clinical risk assessment procedure before they are allowed to be marketed. The CE sign on the package (Fig. 12.2) shows that the material conforms with the essential requirements of this directive: namely safety, efficacy and quality. In other parts of the world (e.g. the USA, Japan, Australia, South America) similar regulations are in effect. Although for this process the term “clinical risk assessment” is used, it should be noted that a new root canal filling material does not necessarily have to pass clinical testing if the manufacturer assumes that the material is both safe and effective from preclinical and other data (e.g. so-called “historic” data from similar/identical materials that are on the market already and/or have been tested in the past). The dentist should therefore ask the manufacturer for clinical data (see below), because he or she is finally responsible for the selection of the material in the individual patient situation and the patient relies on his or her independent expertise (71). There are so far no official regulations concerning recommended periods for a clinical test of root canal filling materials. In analogy to restorative materials, a time of 1 year for excluding catastrophic failures and a time span of 3–5 years for the final testing may be advisable. Products without a CE mark must not be used in those countries where the aforementioned EU Directive is in effect.
Fig. 12.2 The CE sign on the package shows that the material has passed a risk assessment procedure; note the number that identifies the supervising body (“Notified Body”).

The clinical relevance of in vitro test methods (cell and bacterial cultures) is limited, because they do not take the complex clinical situation of the apical region of a tooth into account (Fig. 12.3). Data from such tests provide basic information on the material and can be used to explain certain clinical reactions, e.g. in relation to extrusion of root canal material over the apex. In isolation, they are not sufficient to show biocompatibility of a material (70).
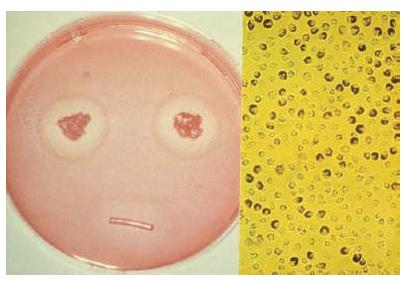
Fig. 12.3 Cytotoxicity test with a polyketone root canal sealer: zone of decoloration around the test specimen (left) indicates moderate toxicity; the partial loss of dye (neutral red) from the cells (right) indicates moderate cell damage.
In vivo biocompatibility test methods are mainly performed on laboratory animals. Relevant tests involve the implantation of a material into the subcutaneous/muscle tissues of rats, mice or rabbits (Fig. 12.4). These tests are mainly designed to test the potential for local toxicity. Of special interest are endodontic usage tests, in which the material is applied as used in the patient, i.e. for filling root canals. With such an approach, special aspects such as apical repair (e.g. new cement formation) or the formation of hard tissue after treatment of teeth with open apices (root-end closure) can be studied, because this requires the interaction of different specialized cell types that so far cannot be simulated in in vitro tests or in implantation studies. Although endodontic usage tests are closer to the clinical situation than in vitro tests, again they have disadvantages. For example the results of end-odontic usage tests depend strongly on the treatment method and there are indications that these tests do not provide a sensitive discrimination between endodontic materials of widely different chemical composition (58).
The allergic potential of dental materials is tested pre-clinically mainly on guinea pigs, which provides a rough estimate. Patients who show clinical symptoms of an allergic reaction to a dental material may be subjected to special allergy tests, which apply a series of materials to the skin (e.g. patch test). Positive patch test results together with corresponding clinical symptoms (e.g. swelling, redness, itching) are indicative of a material-related allergy. For allergy testing and for avoiding relevant allergenic products in the sensitized patient, the composition of the material to be used must be known.
None of the test models described so far for assessing the biological properties of root canal filling materials is identical to the clinical situation under which the material is to be used. Therefore clinical trials are essential; clinical trials seldom allow for histological evaluation, however. This means that the biocompatibility of a new root filling material cannot be evaluated by one test alone (68).
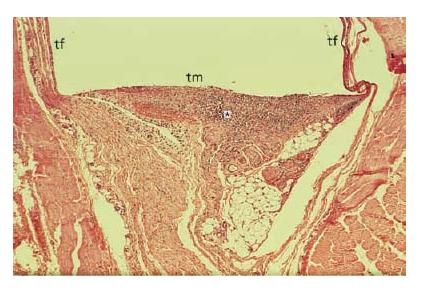
Fig. 12.4 Tissue reaction 14 days after subcutaneous implantation (rat) of a set polyketone root canal sealer filled into a Teflon tube: accumulation of inflammatory cells (mainly polymorphonuclear neutrophilic granulocytes) at the contact area (*) with the test material indicates moderate toxicity; no tissue reaction at the contact area with the Teflon tube. tm = test material; tf = Teflon tube (negative control and material carrier).
Leakage/sealing
It is generally believed that the main cause for failure of endodontic treatment is the lack of seal of the root canal filling (apical and coronal leakage), facilitating bacterial growth. Many studies (about 25% of the current end-odontic literature) are devoted to leakage and sealability. Leakage mainly occurs between the root canal filling and the root canal wall, although there are some reports showing leakage between sealer and core material (guttapercha) and throughout the sealer. Leakage is influenced by the root canal filling material itself and by a number of other factors (Core concept 12.2). The penetration of a sealer into the dentinal tubules is considered to improve the seal (51).
Results reported in the literature on leakage depend greatly upon the test methods being used. Tests most often are performed in vitro and include dye penetration, with additional pressure, centrifugation or vacuum; bacterial penetration and fluid transport are also used (97). The clinical relevance of in vitro studies is questionable and contradictory results have often been reported for the same material using different methods (5). Therefore these tests are – at best – valid in a comparative manner whereby a new material is compared with a clinically established one. In vivo usage tests (e.g. on experimental animals) reveal more relevant results but are more difficult to perform and more uncontrollable variables (e.g. application technique) are included. Again, a set of different test methods is necessary to evaluate the leakage properties of a new root canal filling material. Leakage data reported in the literature for root canal filling materials therefore should be regarded with caution. As with data on other properties (e.g. biological), they are only mosaic tiles that need other information to determine the clinical value of the new material. At present, there is no root canal filling material available which can prevent leakage. Therefore, a bacteria-tight coronal restoration is of critical importance to ensure the success of root canal treatment (99).
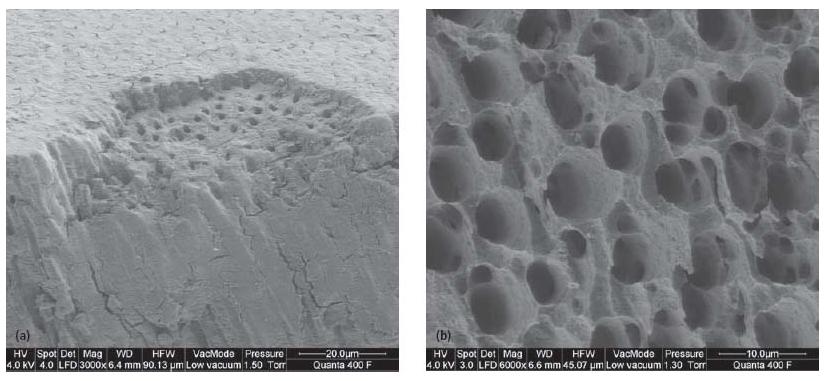
Fig. 12.5 (a) Smear layer on root canal dentin surface after mechanical instrumentation: smear layer partially lost due to fracture of the specimen. (b) Removal of smear layer and erosion of dentin surface after rinsing with 10% citric acid and 5.25% NaOCl.
Gutta-percha cones
Gutta-percha is the most common cone material used for root canal filling. Silver was used in the past but has been abandoned because of the mediocre sealing qualities, even when used together with sealers, and because of high corrosion leading to tooth discoloration and local tissue reactions (Fig. 12.6). Titanium cones are available and have good biocompatibility, but they show low radiopacity and poor adaptation to the root canal wall where the cross-sectional shape of the root canal is not circular. This requires a comparatively high amount of sealer and therefore endangers the seal of the filling. These cones may be considered for use in narrow and curved canals, where the application of gutta-percha points is difficult. Thermoplastic polyester or resin-coated gutta-percha cones have been marketed together with new methacry-late-based sealers (see also Methacrylate-based sealers).
Gutta-percha cones are the material of choice for filling the major part of the canal volume. Gutta-percha cones (even standardized ones) do not fit optimally to the shaped root canal and therefore must be compacted and used together with sealer; the less sealer necessary, the better.
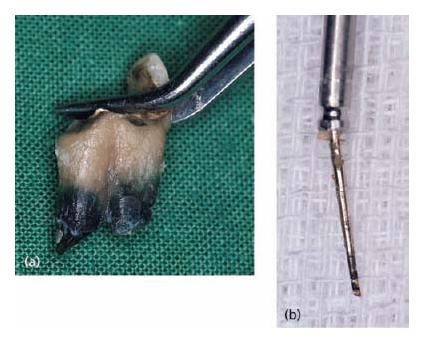
Fig. 12.6 (a) Discoloration of a root after root canal filling with a silver cone. (b) Removed silver cone showing signs of severe corrosion.
Composition
Gutta-percha is a natural product that consists of the purified coagulated exudate of mazer wood trees (Isonandra percha) from the Malay archipelago or from South America. It is a high-molecular-weight polymer. Two forms of gutta-percha are relevant for dental products: the α- and the β-form. The β-form is used in most gutta-percha cones (less brittle than the α-form) but the α-form is used for injectable products because of its better flow characteristics.
The composition of gutta-percha cones (Table 12.1) varies considerably between manufacturers. This and the fact that gutta-percha is a natural product may be the reasons for the different properties reported for different brands. Formerly, cadmium (Cd)-based dyes were added to provide a yellow color, which should facilitate removal (if necessary, e.g. for revision). Modern gutta-percha preparations use other colorants and do not contain any intentionally added Cd compounds. Some gutta-percha preparations contain calcium hydroxide or chlorhexi-dine, with the aim of enhancing their antibacterial activity (temporary root canal dressing) and thereby stimulating apical healing. Clinical experience of such additions is so far limited.
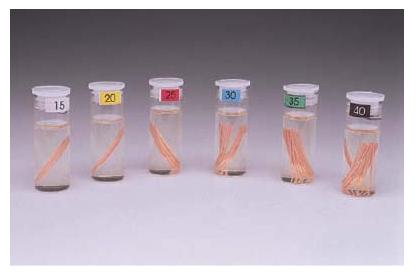
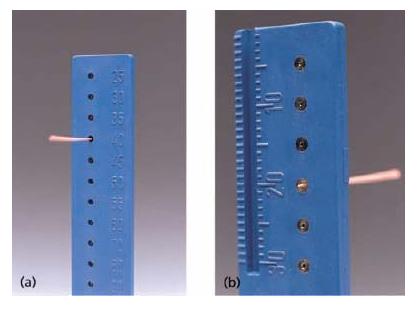
Gutta-percha cones are supplied by the manufacturers in different sizes (length, diameter, taper; Table 12.2). Standardized cones are frequently used and the idea of having a cone that corresponds closely to the shape and the dimensions of the prepared root canal is striking. However, there are discrepancies between the shapes of the cones and the shaping instruments (Fig. 12.7), and the actual dimensions of the gutta-percha cones may show considerable variation. Therefore, it is advisable to check the dimensions of each cone, e.g. by a suitable gauge, prior to use (Fig. 12.8). Some manufacturers offer gutta-percha cones with a color coding according to the ISO system for the different sizes (ISO 10–ISO 140) (Fig. 12.9). Cones with a 4% or 6% (and up to 12%) taper are offered in sizes using the ISO numbering system (i.e. 10–140); gutta-percha cones with the same dimensions as special root canal shaping instruments with varying taper are available.
Table 12.1 Typical composition of gutta-percha cones.
| Components | Composition (%) |
| Zinc oxide | 66 |
| Metal sulfates (radiopacity) | 11 |
| Gutta-percha | 20 |
| Additives like colophony (rosin, mainly composed of | 3 |
| diterpene resin), pigments or trace metals |
Table 12.2 Dimensions of gutta-percha cones.
| Type of cone | Size |
| Standardized cones | Corresponds in diameter and taper (2%) to root canal |
| shaping instruments according to ISO 6877. The sizes | |
| of the gutta-percha cones range from ISO 10 to ISO | |
| 140 (Fig. 12.9) | |
| Accessory cones | Larger taper, descriptive size, may be used for lateral |
| compaction | |
| Greater taper cones | Cones with a 4% or 6% (and up to 12%) taper or |
| cones with varying taper used together with special | |
| engine-driven root canal shaping instruments (see | |
| Chapter 11) | |
| Compaction cones | Taper corresponds to the taper of finger-spreaders |
Fig. 12.7 Scanning electron microscope picture of the tip of a gutta-percha cone and the corresponding root canal file; note the discrepancies in shape.
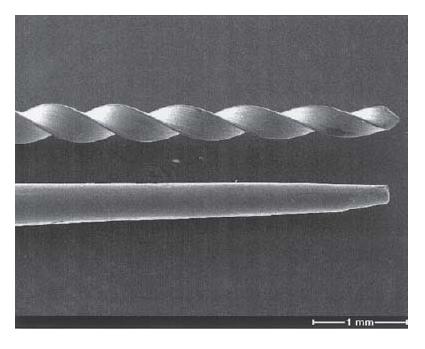
Fig. 12.8 (a) Gauge for controlling the size of the actual gutta-percha cone. (b) The actual cone is too thin, because it reaches out of the gauge.
Gutta-percha may be used cold in combination with a sealer. Owing to its thermoplastic properties, guttapercha may be used also in a heated state, which allows closer adaptation to the canal walls (Fig. 12.10). The products consist of a plastic core (carrier) coated by α-form gutta-percha for improved flow characteristics and to reduce shrinkage after cooling. Gutta-percha also may be liquefied at 70°C (Ultrafil) or 160/200°C (Obtura II) and injected directly into the root canal (see Chapter 13).
Technical properties/leakage
Gutta-percha cones are flexible (elastic) at room temperature, become plastic at about 60°C and are volume constant under mouth conditions. Heating leads to expansion (and cooling to contraction), a fact that reduces the sealing quality of warm or liquid gutta-percha application (when used without a sealer). Gutta-percha is soluble in organic solvents such as eucalyptus oil.
Fig. 12.9 Scheme for the dimensions of a standardized gutta-percha cone according to ISO 6877; d1 × 100 = size designation of guttapercha cone (ISO 10–ISO 140).
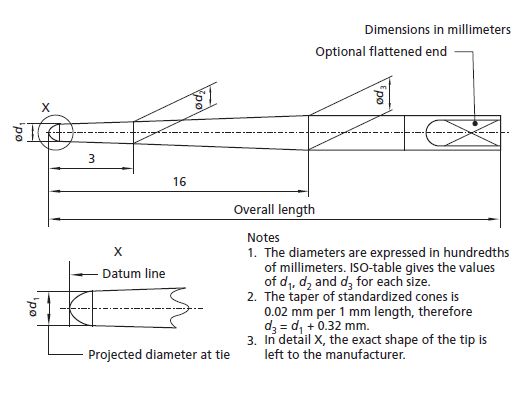
Fig. 12.10 (a) Oven for warming gutta-percha cones; (b) and the corresponding cones.
Gutta-percha does not adhere to the canal walls, regardless of the filling technique applied, resulting in the potential for marked leakage. Therefore, it is generally recommended that gutta-percha (used cold or heated) is used together with a sealer. For an optimal seal the sealer layer should generally be as thin as possible, therefore the skill of the operator plays an important part in the success of the treatment by correctly compacting gutta-percha; it is apparently of minor importance which method of compaction is used.
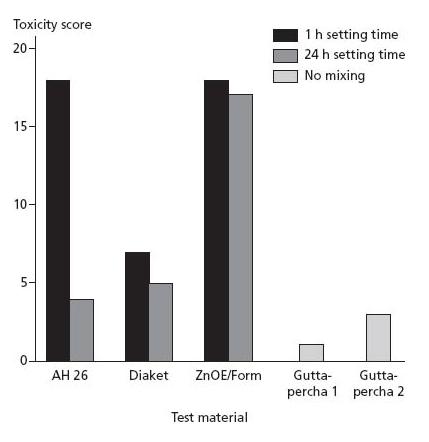
Fig. 12.11 Cytotoxicity of different root canal filling materials; human cells were exposed to eluates of the materials and the effect upon cell growth was measured; high scores indicate strong cytotoxicity. For sealers, effects of freshly mixed materials and set materials were measured; for gutta-percha, two brands were tested. ZnOE/Form = formaldehyde-containing ZnOE sealer (69).
Biological properties
No systemic toxic reactions toward gutta-percha have been reported in the literature. Allergic reactions to gutta-percha are extremely rare. One case was reported of a suspected allergic reaction during a root canal treatment with a patient who was sensitized to natural latex. No latex gloves were worn during treatment, but pain, swelling of lips and diffuse urticaria developed after treatment. After 4 weeks the gutta-percha cone was removed and the symptoms abated. The allergy was attributed by the authors to the fact that pure guttapercha and natural latex are fabricated from natural substances derived from trees of the same botanical family (11). No further cases have been reported. Cones made from synthetic gutta-percha are available.
Depending on the product, several cell culture studies have demonstrated gutta-percha to have little or no cytotoxicity (Fig. 12.11). Generally, gutta-percha is well tolerated by animal tissues (e.g. rat and mouse connective tissue); it induces the formation of a collagenous capsule with no or almost no inflammation (Fig. 12.12).
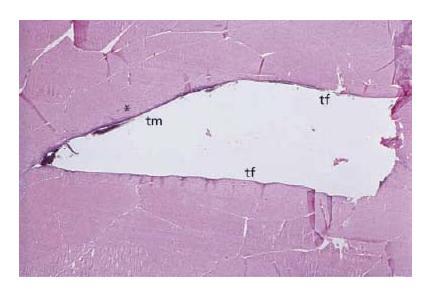
Fig. 12.12 Tissue reaction 7 days after intramuscular implantation of guttapercha: no inflammatory cells can be observed at the contact area with the test material (*), which indicates good biocompatibility. tm = test material; tf = Teflon tube (negative control and material carrier).
The elevated temperatures involved in the application of injectable liquefied gutta-percha or of heat-mediated condensation/compaction techniques have been the motive for several investigations into the involved risk for adverse clinical effects. Intracanal temperatures have been measured, the highest being for the thermo-mechanical condensation technique (see Chapter 13) (Table 12.3). Interestingly, for liquefied gutta-percha (Obtura II), which is heated to more than 160°C, the intracanal temperature shows a maximum of only 61°C, which reflects the cooling process during application (94).
However, the main target tissue (the periodontal ligament) is separated from the heated gutta-percha by dentin, which, owing to its low thermal conductivity, acts as a thermal isolator. Its effectiveness depends on the dentin thickness. Therefore temperature measurements at the surface of the root are clinically more relevant. It is generally accepted that a temperature rise of approximately 10°C above normal body temperature is critical if maintained over 1 min; over 5 min bone damage will occur (23). Again, the highest temperatures were measured on the root surface with the thermomechani-cal compaction technique, with differences depending on the rotational speed of the compacting instrument. After stopping compaction, heat dissipated in 15–30 s for an elevation of less than 10°C (66).
The reaction of the target tissues (periodontal ligament) after injection of heated gutta-percha into the root canals of a dog showed no evidence of inflammation. In the case of overfilling, an acute inflammatory reaction was observed briefly after insertion and a chronic/foreign body reaction was found in long-term experiments (46). The classical warm vertical condensation or the warm lateral condensation technique did not cause any heat-related periodontal damage in monkeys and miniature pigs. Contrary to these data, thermomechanical compaction of gutta-percha with a sealer caused tissue damage (see Key literature 12.1).
In conclusion, for melted injectable gutta-percha, no tissue damage is expected due to rapid cooling during application and the isolating capacity of the dentin layer. If this layer is not present, e.g. after overfilling, a tissue reaction may occur. No such risks exist for the classical warm condensation technique, with the use of heated instruments or with the prewarming of gutta-percha cones. The use of sealers further reduces tem perature rises. However, with the thermomechanical compaction technique elevated temperatures on the root surfaces have been recorded, as well as tissue damage with cementum resorption and ankylosis.
Table 12.3 Temperature measurements for liquefied gutta-percha.
| Technique | Intracanal | Tooth surface |
| temperature | temperature rise | |
| (°C) | (°C) | |
| Ultrafil | 70 | |
| Obtura II | Max. 61 | Max. 8.9 |
| Warm vertical condensation | 45-80 | 3-7 |
| Thermomechanical compaction | 55-100 | 14-35 |
Antimicrobial properties
Gutta-percha provides some antimicrobial properties, with the active substance being ZnO from which zinc ions (Zn2+) are mobilized by hydrolysis. Some brands of gutta-percha are active against anaerobically cultivated isolates from root canals. The occurrence and the size of the inhibition zones varied with the bacteria used for testing and the brand of the gutta-percha cone (93).
Handling properties
Gutta-percha cones are usually supplied by the manufacturer in a non-sterile form. Storage in commonly used disinfectants may have a negative influence on the mechanical properties of the cones and should be avoided, unless evidence is presented that the cones are not damaged. Instead, an effective surface disinfection (e.g. with 5.25% NaOCl) immediately prior to use is advisable; afterwards the cones should be rinsed in 70% alcohol to prevent NaOCl crystals forming on the gutta-percha cone. Recently, gutta-percha cones that are “free of living germs” (declaration of the manufacturer) have been marketed (Fig. 12.13). Gutta-percha cones should be stored in cool and dark conditions in order to prevent hardening and brittleness due to further crystallization and/or oxidation. A technical problem with the use of heated gutta-percha is the higher frequency of extrusion of root canal sealer.
Owing to its comparatively soft consistency, guttapercha can be removed mechanically by conventional hand file or by rotary instruments (see Chapter 20). Gutta-percha preparations using a plastic carrier can be removed using organic solvents, e.g. eucalyptus oil. The carrier can be bypassed by endodontic instruments. The radiopacity of gutta-percha was measured to be between 6.14 and 8.8 mm Al (76) and this is considered to be sufficient.
Fig. 12.13 Gutta-percha cones delivered (“germ-free”) in an aqueous solution of ethanol and hexetidine.
Sealers
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses