Fig. 6.1
Translational Evidence Mechanism: transforming scientific evidence into better consumer choices
For any discipline-centered evidence-based decision-making, this mechanism consists of three components: Translational Evidence Organization, central database, and decision-making algorithm. The outcome of this mechanism is to provide end users – patients or clients – with current, best evidence to make informed and optimal decisions regarding life choices, choices specific to any discipline in which consumer-based wants, needs, and desires are expressed.
The product of such a system is typically called a guideline that takes into account the probability (or odds ratio) of an event or events, the human preferences (expressed as benefits or trade-offs) attributed to these events, and their costs. Similarly, researchers and policy-makers may be granted access to evidence for future research or policy needs.
Evidence regarding the “average patient” is helpful to researchers investigating current issues of discovery, to decision-makers in developing policy of sociopolitical and socioeconomic importance, monitoring health care systems including private practice, and determining care and cost-effective therapies and treatments. Best evidence assists by protecting society in reducing health inequalities and potentials for harm.
6.2.3 Translational Evidence Organization
The Translational Evidence Organization develops, verifies, maintains, and updates current, best evidence for end users in their consultations with service providers. This organization (Fig. 6.2) requires a workforce and input process that initiates an inquiry regarding uncertainties in consumer decision-making.
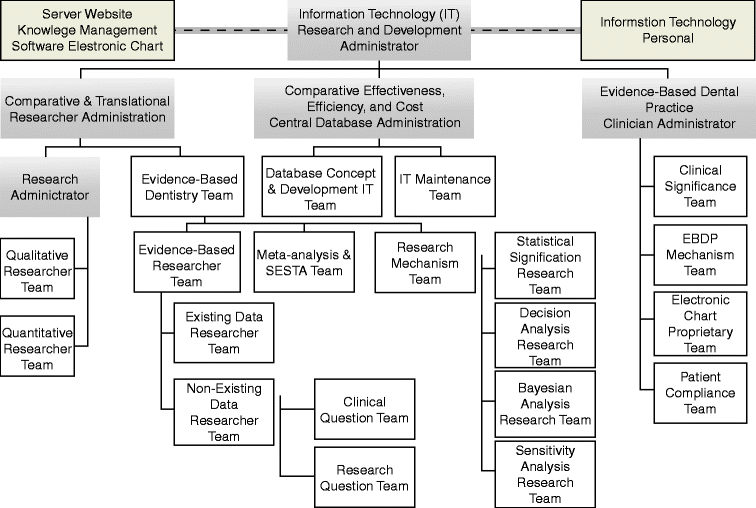

Fig. 6.2
Translational evidence organization and manpower
This inquiry drives this organization to provide in real-time, effective, and efficient decision-making; in other words, in a manner that demands current best evidence for immediate decision-making in determining the optimal cost/benefit choice for the consumer. If best evidence does not exist currently, the organization initiates an internal inquiry to develop new evidence-based on consumer-based wants, needs, and desires.
The primary purpose of the Translational Evidence Organization is to arbitrate published evidence and, in its absence, identify research that needs to be conducted by organizational affiliates or the research community in producing needed data. The Research Administrator and Evidence-Based Dentistry Team are responsible for this purpose.
The Comparative and Translational Researcher (Translational Researcher) coordinates with IT Research and Development administrator to vest a central database with evidence defined by templates of inputs and outputs that may be stratified by geographic, discipline, population, and/or other categorical needs. Outputs consist of arbitrated decision data produced by the quantitative researchers, including related utility (attitudes, beliefs, and preferences of both providers and consumers), produced by qualitative researchers, and cost data.
Arbitrated best evidence meets the rigorous standards of statistical significance, the soundness and generalization of information, or whether findings can be applied to similar consumers in similar settings. Outputs are stated in terms of validity and reliability of study design to express confidence in providing best evidence.
For health care, all are displayed in clinical practice guidelines (CPGs). The CPG is the vehicle, or professional standard, that manages data for use in clinical practice.
Additional post arbitrators of best evidence include the provider (clinician) and consumer (patient). The clinician is concerned with clinical significance – whether research findings can be applied to individual patients. Dental clinicians, dentists, make judgments that may weight best evidence differently from researchers. Initially, clinical significance of decision data is coordinated with dentists in developing nationally, regionally, or locally relevant best evidence.
The dentist is provided a CPG with decision and utility best evidence and locally provided cost schedules. The dentist provides an assessment of the clinical significance of the CPG based on practice and local factors. This assessment is developed from applying knowledge logically based on concepts learned during training and implicitly in rendering health services based on experience and patient characteristics of well-being.
During care delivery, dentists perform assessments, evaluate services needed, and develop plans for treatments and therapies. In providing dental care services, the dentists may contribute to the understanding of the “when, where, and how” of knowledge. The dentist’s evaluation of clinical significance is used by the translational researcher to reject or modify the clinical practice guideline or to re-identify and conduct investigations that produce other clinically relevant data.
Personal and professional experiences, values and preferences, and appropriate practices, as well as patient well-being, quality of life (QoL) issues, and costs, weigh heavily on whether best evidence is used in clinical decisions. Thus, dentists are able to predict clinical outcomes in weighing risks against benefits and costs for individual patients. Dentists may also make relevant standards of care in their local practice and for specific patient population characteristics. These clinical validations are necessary to translate research data into clinically useful data for patient care.
Clinical significance also addresses the importance of the evidence that takes into consideration the long-term multifaceted monitoring of evidence in the context of human behavior (patients). However, clinical significance may vary between dentists and between patients. This difference results because dentists, as well as patients, make judgments that weigh differently personal and professional experiences, values and preferences, and appropriate practices.
In other words, judgments of risk and benefits vary because of differences in weights given to values and preferences that also include costs. All is important for patients in accepting best evidence in their acquiring the highest level of cost-effective services, either through fee-for-service or as a defined benefit of their dental insurance plan.
6.2.4 Patients as Consumers
Patients are typically categorized as the consumers of products and services and not the developers or guarantors of knowledge. Patients become the “conceptual subject” to which best evidence is applied and quantitative and qualitative outcomes are measured.
However, patients may be advocates or adversaries of evidence. Patients may also exert demands on evidence to meet specific, personal needs. They may exert influence on the development and application of knowledge that does not necessarily meet acceptance criteria of researchers and dentists, but serves a personal need.
They may also exert pressure to deny the development and application of knowledge that is contrary to their philosophical beliefs. Even in the profession’s best efforts of informing patients with best evidence and using clinician expertise to communicate individualized, effective treatments, patients ultimately decide if treatment regimens are adhered to or rejected outright. Patient adherence, modification, or rejection of best evidence in treatment scenarios provides the translational researcher with its meaning in practice.
The translational researcher uses these evaluations in updating clinically relevant data. These updates are processed using decision, Bayesian, and sensitivity analyses within the central database.
Thus, the translational researcher is the final arbiter of evidence. The translational researcher functions, simplistically, to translate basic research language into the language of the clinician and patient. In other words, basic research is assessed, evaluated, and disseminated to the clinician in a means usable for shared decision-making.
The product of evidence-based dentistry is the published clinical practice guideline. While there is no requirement that the published clinical practice guideline be implemented in private practice, dentists could use best evidence to offer their patients best practices in a state-of-the-art organization and facility. This requires that dentists review and effect personal, behavioral changes to accomplish care and service advances.
6.3 Central Database
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


