Fig. 11.1
Odontoblasts and Hoehl’s cells are located at the periphery of the pulp
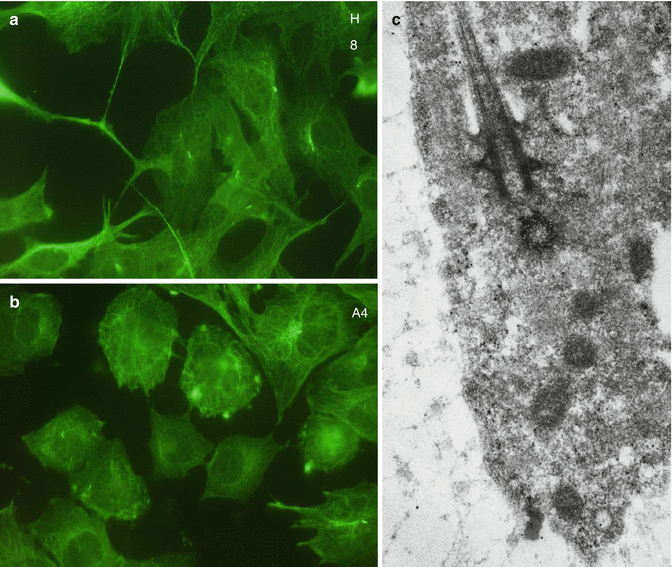
Fig. 11.2
Immunohistochemical visualization of alpha acetyl tubulin, a component of microtubules, in H8 (a) and A4 (b) cell lines. In c = a pulpoblast displays a cilium and basal body
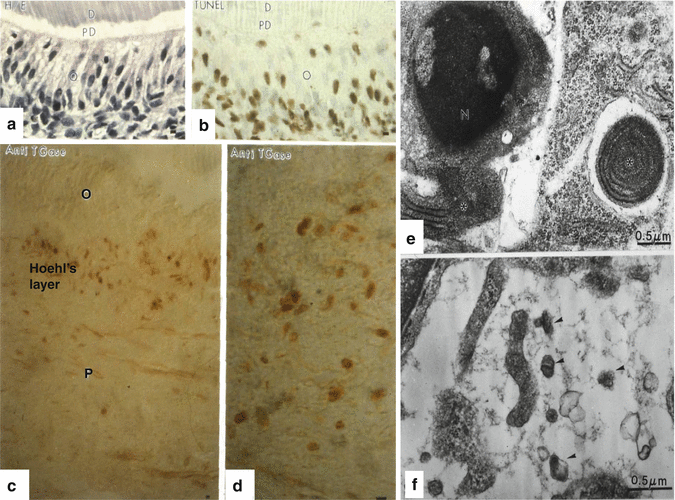
Fig. 11.3
(a, b) (a) Hematoxylin-eosin staining of a control semi-thin epon section. b: after TUNEL staining apoptotic cells (colored in dark brown) are located in the odontoblast (o) layer. PD predentin, D dentin. (c, d) An antibody raised against the apoptotic marker the anti-transglutaminase (anti-TGases) stains cells located in the Hoehl’s layer, but not odontoblasts (O) or pulp (P) cells. (e) Chromatin condensation in the nucleus (left part of the figure) and isolated rough endoplasmic reticulum (asterisk) inside another pulp cell (right part of the figure). (f) Apoptotic bodies (arrowheads) after the apoptotic destruction of the cell
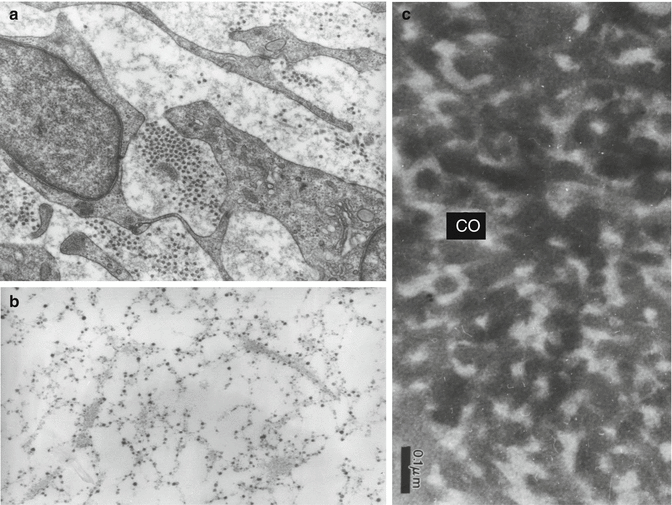
Fig. 11.4
(a) Pulpoblasts and collagen fibrils displaying different diameters. (b) Histochemical staining of glycosaminoglycans appearing as electron-dense granules aligned along collagen fibrils and pulpoblast processes. (c) Alcian blue stained predentin after rapid freezing freeze substitution. The collagen fibrils appear electron lucent; intercollagenic spaces are electron dense and stained by the cationic dye
Type I collagen is the major fibrous component of the dentin matrix, but pulp matrix also contains significant amounts of type III collagen (Figs. 11.4, 11.5, and 11.6). Fibronectin and proteoglycans are also present in the dental pulp (osteoadherin/osteomodulin) (Linde 1985). Type III collagen constitutes 28 % of the pulp (Shuttleworth et al. 1978). It may form up to 42.6 % type III of the total collagen (see table 11.1, 41 %). Type V collagen comprised two different molecular species of [a1(V)]2 a2(V)a2 (V)a3(V), the ratio of which represented, respectively, 56,41 and 2 % of the total collagen (Tsuzaki et al. 1990). Type VI (0.5 %) is associated with microfibrillin.
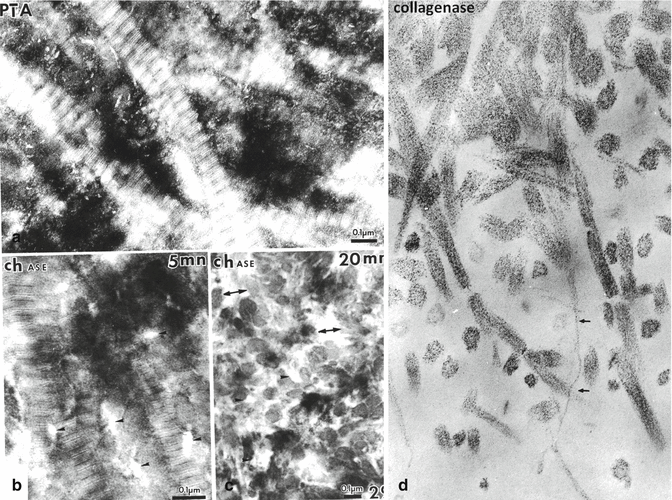
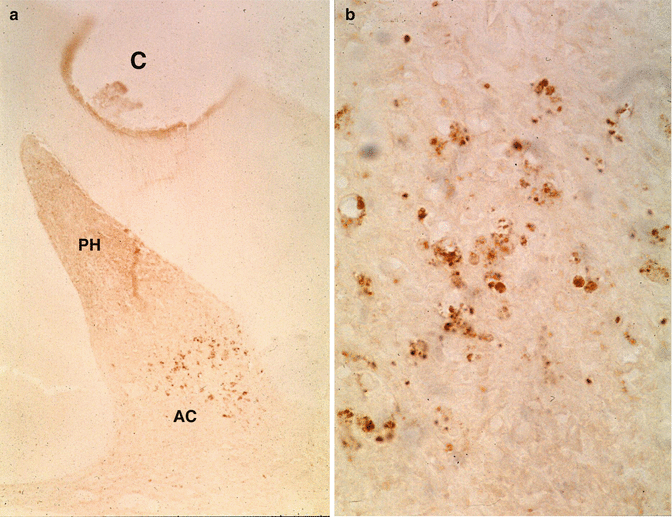

Fig. 11.5
(a) Dentin stained by phosphotungstic acid (PTA). The periodic banding of the collagen fibrils is clearly seen. Along the surface of collagen fibrils, and in the intercollagenic spaces, PTA positive intercollagenic spaces are densely stained. (b) Treatment of the section with a chondroitinase for 5 min reduce partially the PTA staining, whereas in (c): enzyme digestion with chase for 20 min largely suppresses the proteinaceous material located in intercollagenic spaces. Treatment of the sections with a bacterial collagenase disrupt the fibril in ¾ and ¼, (small arrows), and in thickness

Fig. 11.6
(a) After the preparation of a cavity (C) and accumulation of cells in the pulp horn (PH), apoptotic cells (AC) are grouped in the lower part of the mesial pulp horn. (b) Larger magnification shows pulp cell fragmentation of apoptotic cells
Table 11.1
Composition of the pulp ECM
|
Component
|
Protein family
|
Specific protein
|
|---|---|---|
|
Collagen
|
Collagens
|
Type I 56 %
Type III 41 %
Type V 2 %
Type VI 0.5 % associated with microfibrillin
|
|
Noncollagenous proteins
|
Phosphorylated ECM proteins
|
DSPP, DPP, DSP, bone sialoprotein, osteopontin, MEPE
|
|
Non-phosphorylated proteins
|
Fibronectin, osteonectin
|
|
|
Proteoglycans–glycosaminoglycans
|
Chondroitin sulfate-4 (CS-4) and CS-6 (60 %), dermatan sulfate (DS) 34 %, keratan sulfate (KS) 2 %, hyaluronic acid
|
|
|
Growth factors
|
BMPs, type 1A and II receptors for TGFβ, activin
|
|
|
Proteins taking origin from the plasma
|
Fibronectin
|
|
|
Enzymes
|
Metalloproteinases (collagenases, gelatinases, stromelysine-1; tissue inhibitors of MMPs (TIMPs), alkaline and acid phosphatases, catalytic lysosomal and extracellular enzymes
|
|
|
Phospholipids
|
Membrane and ECM phospholipids (proteolipids)
|
Collagen degradation is regulated by matrix metalloproteinases (MMPs). TNF-α, ΙΛ − 1β, and ΙΛ − 6 ανδ ΤΓΦ − β1 πλαψ ρολε ιν χολλαγεν δεγραδατιον are mediated by pulp fibroblasts (Wisithphrom and Windsor 2006) (Fig. 11.6).
Phosphorylated and non-phosphorylated proteins: Osteocalcin, dentin sialophosphoprotein (DSPP), and matrix extracellular phosphoglycoprotein (MEPE) have been detected in pulp cell cultures. DSPP and MEPE expressions are regulatory pattern of DPCC with stem cell characteristics. DSPP is processed by protease (BMP-1) into three major domains: dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP). Expression of full-length Dspp mRNA by quantitative real-time polymerase chain reaction analysis was significantly higher in odontoblasts than in pulp (Yamamoto et al 2015). DSPP-derived proteins in porcine pulp are expressed at both the protein and mRNA levels.
Tenascin and fibronectin were found by immunohistochemistry at the periphery of the pulp, next to the odontoblasts of normal human dental pulp.
Glycosaminoglycans (GAGs) and proteoglycans (PGs) are present in the dental pulp. GAGs are formed by chondroitin sulfate, dermatan sulfate, heparan sulfate, keratan sulfate, and hyaluronic acid. CS-4 and CS-6 are the major glycosaminoglycans, hyaluronic acid and keratan sulfate being presented in minor amount (Rahamtulla 1992).
Versican, a proteoglycan aggregate, has also been extracted from the dental pulp. Versican, hyaluronan, and link protein form ternary aggregate structures in the rat dental pulp (Shibata et al. 2000).
Metalloproteinases (MMPs) and tissue-specific inhibitors (TIMPs) are implicated in the extracellular matrix degradation (Sulkala et al. 2004). cDNA microarray demonstrated the high level for MMP-13 (collagenase-3) and a lesser expression of MMP-16 (MT3-MMP) and TIMP-1, especially during caries progression. During rat tooth eruption, a disintegrin and metalloprotease with thrombospondin type 1 motifs (ADAMTS) is implicated in cleaving proteoglycans such as aggrecan, versican, and brevican. ADAMTS1, ADAMTS4, ADAMTS5, and versican were expressed in dental pulp cells (Fig. 11.5). Dental pulp cells are involved in both production and degradation of versican with secreting ADAMTS1, ADAMTS4, and ADAMTS5 (Sone et al. 2005).
11.2 Cells
The pulp periphery: At the outer border of the pulp, odontoblasts and the so-called Hoehl’s cells form continuous layers. These postmitotic cells have the capacity to undergo terminal differentiation. Odontoblasts are implicated in the synthesis of collagen and noncollagenous extracellular matrix components. Some ECM proteins are phosphorylated (SIBLINGs), whereas others are non-phosphorylated. ECM components are implicated in predentin and dentin formation, followed by dentin mineralization. Due to a fixation artifact, the formation of a cell-free layer results in from fixation and dehydration. A cell-free area underlines odontoblasts and Hoehl’s cells, which do not appear on sections after adequate fixative perfusion. Fenestrated capillary loops infiltrate the layer formed by odontoblasts and Hoehl’s cells but do not cross the terminal junctions located between the distal odontoblast cell bodies nor penetrate within the predentin. In contrast, axons infiltrate the odontoblastic layer and penetrate into the predentin. A few axons penetrate into dentin tubules and occupy the inner dentin but are found only in the inner 150 micrometers (Fig. 11.1).
Pulp cells are present in the dental pulp. Fibroblasts (pulpoblasts) and fibrocytes are the prominent cells, with variable cell density. They are elongated, with thin spinous processes. A few macrophages, plasmocytes, mast cells, and leukocytes have been also identified. Pulpoblasts contain dense cytoskeletal proteins, including microfilaments, intermediary filaments, and microtubules. Immunostaining of alpha acetyl tubulin revealed the presence of microtubules (Fig. 11.2a, b) associated in cilium and basal corpuscle (Fig. 11.2c).
Capillaries connect arterioles and venules. Capillaries display continuous thick or thin endothelial lining. Fenestrated endothelial cells contribute to the control and balance between the intravascular compartment and the interstitial tissue. Lymphatic capillaries have been recognized at the pulp periphery. Beneath the odontoblast compartment, the so-called Hoehl subodontoblastic compartment constitutes a second layer of cells, which may differentiate and become a second generation of odontoblasts. The renewal of odontoblasts requires newly differentiated cells, and because odontoblasts are postmitotic cells, there is a need for mesenchymal cells taking origin in the stromal pulp.
11.3 Neuropeptides in Dental Pulp
Nerve fibrils penetrate into the pulp within the apical region. They are surrounded by a myelin sheath that surrounds the axon. The role of neuropeptides, including substance P, calcitonin gene-related peptide, neurokinin A, neuropeptide Y, and vasoactive intestinal polypeptide has been discovered. Neurotransmitters or neuromodulators presumably play a variety of functions, participating in paracrine, endocrine, and neurocrine forms of communication (Caviedes-Bucheli et al. 2008).
11.4 Human T-Lymphocyte Subpopulation
Pulp inflammatory cells include polymorphonuclear neutrophilic leukocytes and mast cells implicated in the defense mechanisms. Interleukin-8 expression is elevated in the irreversibly inflamed dental pulp, but lacking in the normal caries-free pulp (Huang et al. 1999).
CD45+ represented 0.94 % ± 0.65 % of cells obtained from the enzymatic digestion of the whole dental pulp cells. CD16 + CD14+ granulocyte/neutrophils (50.0 % ± 9.08 % represent the major subpopulation in CD 45+ cells, followed by CD3 T lymphocytes (32.58 ± 11 %), CD14+ monocytes (8.93 % ± 5.8 %), and HLA-DR highLin1+ dendritic cells (4.51 % ± 1.12 %). Minor subpopulations included CD3-CD56+ natural killer cells (2.63 % ± 1.15 %) and CD19 B lymphocytes (1.65 % ±0.89 %). In addition cells presenting a phenotype compatible with Foxp3/CD25 are seen in regulatory T lymphocytes (Gaudin et al. 2015).
Pulp cells are present for a limited period of time. Odontoblasts (Fig. 11.3a, b) are postmitotic cells that survive the initial period. Later, the so-called Hoehl’s cell layer becomes apoptotic and displays a turn over more rapidly than the odontoblasts (Fig. 11.3c, d). Within the dental pulp, some pulpoblasts underwent apoptosis; their nuclei display condensed chromatin (Fig. 11.3e). Cytoplasmic inclusions contribute to the restricted aging of pulpoblasts. After the complete degradation of pulp cells, apoptotic bodies are present in the pulp extracellular matrix. They are destroyed by phagocyte or by macrophages (Fig. 11.3f). Apoptosis contributes actively to the pulp defense mechanisms.
11.5 The Carious Pulp
Carious pulps can be classified as being at the transitional stage, or displaying partial pulpitis, or total pulpitis and/or total necrosis (Di Nicolo et al. 2000).
If caries progresses slowly, there is time to form reactionary dentin or tertiary dentin (Bjorndal 2008). Pulp can be affected or infected. Bacteria are rarely seen in the unexposed pulp, whereas they are often sees in infected and necrotic pulps (Massler and Pawlak 1977). Microorganisms can reach the pulp via the dentin tubules. Often apoptotic pulpal cells at some distance from cavity preparations may display fragmentation of nuclei (Fig. 11.6a, b).
Such rapidly progressing carious lesions lead to the formation of an atubular dentin or to the complete absence of tertiary dentin. This rapidly progressing lesion leads to pulp necrosis and furthermore to periapical lesion formation. The formation of reactionary or reparative dentin is representative of the pulp response to the carious lesion. Dentin sclerosis, dead tracts, or reparative dentin are correlated with sex, age, and the type of surface location of the lesion (Stanley et al. 1983). Dentin sclerosis results from aging. The formation of translucent zone is observed in response to caries of the slow type and other mild irritations. The production of reparative dentin is directly related to the carious decay. Slowly progressing caries may become arrested caries lesions, with occlusion of the tubules by mineral deposits contributing to the formation of a “transparent zone” subjacent to the mineralized carious dentin. In this zone needle and rhombohedral crystals have been identified, together with hydroxyapatite and whitlockite crystals. They occlude the lumen of the tubules (Fig. 11.8a, b).
A gradual degradation of the dental pulp is seen. The density of the odontoblast layer slowly decreases (Fig. 11.7a), and finally, between the mineralized dentin and the pulp, an empty border is gradually inhabited.
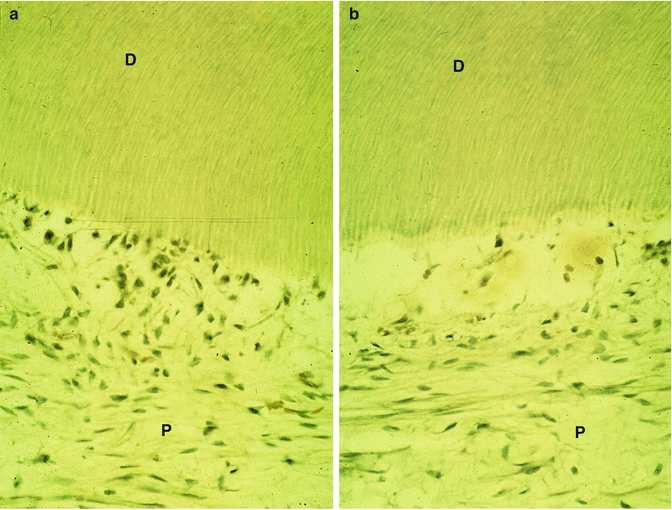

Fig. 11.7
(a) The preparation of a cavity induces disturbances of the odontoblast layer. P pulp, D dentin. In (b): the layer of odontoblast is empty. D dentin, P pulp
11.6 Reactionary and Reparative Dentin Formation
Odontoblasts are postmitotic cells. After development of carious lesions, under pro-inflammatory stimuli, dental pulp cells can differentiate and produce reactionary or reparative dentin. The pro-inflammatory cytokine tumor necrosis factor α (TNFα) may be a mediator involved in dental pulp cell differentiation toward an odontoblastic phenotype. TNF-α-stimulated pulp cells display increased expression of DPP, DSP, DMP-1, and osteocalcin. The TNF-α differentiation of dental pulp cells toward an odontoblastic phenotype occurs via p38 and is negatively regulated by MMP-1 expression (Paula-Silva et al. 2009). Although there are no specific odontoblastic markers, osteocalcin, osteonectin, alkaline phosphatase, bone sialoprotein, and DSPP have been used as indicators of odontoblastic differentiation. Studies have shown that MMP-2 and MMP-9 inhibition is necessary for dentin matrices to mineralize alters dentin remodeling (Fanchon et al. 2004).
Bacterial invasion occurs in dentin that is stained bright red by 0.5 % basic fuchsine – propylene glycol solution, whereas caries-affected dentin that is bacteria-free stains pink. Collagen fibrils in infected dentin have lost their cross-banded appearance in transmission electron micrographs, indicating they are irreversibly denatured (Kuboki et al. 1977).
Reactionary dentin and reparative dentin are both strongly immunopositive for osteopontin (OPN), a phosphorylated protein of the SIBLING family, also implicated in intracellular cell signaling and inflammatory process (Aguiar and Arana-Chavez 2007). DMP-1 and collagen were associated and seem to be essential for reactionary and reparative dentin formation (Aguiar and Arana-Chavez 2010) (Figs. 11.6, 11.7, 11.8, 11.9 and 11.10).




