The mineralization process
Publisher Summary
Hydroxyapatite crystals are usually confined to areas where there is a need for high mechanical strength, rigidity, or hardness as in bone, dentine, and enamel. They do not normally occur at all in soft tissues, the only other site of occasional local occurrence being within such epithelial keratins as hard claws and beaks. The restriction of hydroxyapatite crystals to certain kinds of tissue suggests that some mechanism exists for their deposition in tissues where they are functionally useful and nowhere else. The nature of this mechanism is still not completely understood. An idea that persists is that one or more of the organic components of hard tissues is specifically concerned with deposition of minute apatite crystals, and this is sometimes referred to as the organic matrix or nucleator of the crystallites. The term “matrix” is also often used more loosely in a histological context to denote a continuum that fills spaces between discrete entities. Thus, the continuous material, rich in proteoglycans and collagen, that fills the spaces between the cells of cartilage is referred to as the cartilage matrix, despite the fact that the cells have themselves produced this intercellular material rather than been produced by it.
An early theory of mineralization – the alkaline phosphatase hypothesis
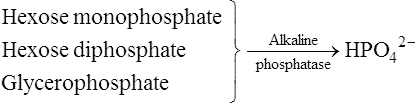
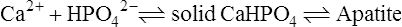
A number of objections raised to this theory led to its gradual abandonment. Firstly, it was argued that the concentration of organic phosphate in plasma was too low for this to serve as an effective source of phosphate ions. Robison conceded this point but suggested that a store of organic phosphate was built up at the calcification site by the alkaline phosphatase acting in reverse, before calcification began. A second criticism was that some other sites, such as the kidney, which do not normally calcify, contain considerably higher concentrations of alkaline phosphatase than the calcification sites themselves. Thirdly, it was pointed out that the inorganic phase is more highly organized than can be accounted for by simple precipitation, since the apatite crystallites are regular in size, distribution and orientation. To answer this, Robison proposed a ‘second mechanism’, necessary for smooth deposition of inorganic crystals, which partly anticipated the later epitactic concept (page 456). Finally, it appeared that there is no necessity for the phosphate ion concentration to be raised for deposition of solid calcium phosphate to occur. Slices of cartilage from rachitic rats became mineralized when incubated in solutions containing the same concentration of calcium and phosphate ions as those found in the serum of young normal rats. The same conclusion can be reached on the theoretical grounds discussed in the following section.
Solubility product of some calcium phosphates
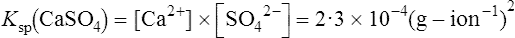
Biological apatite, the crystalline phase of bone, dentine and enamel, consists essentially of hydroxyapatite modified by the presence of carbonate, small amounts of magnesium, sodium, and possibly citrate, either within the lattice or adsorbed on the crystal surfaces (page 430). The theoretical possibilities for ionization of hydroxyapatite are complex because more than one calcium ion and more than one phosphate ion can arise per unit of Ca10(PO4)6(OH)2. In addition the pH (which controls hydroxyl ion concentration), the carbonate concentration and the presence of macromolecules all affect the solubility product. The concepts used to describe the solubility characteristics of simple salts are therefore not easily applied to biological apatite. Furthermore, the formula given for hydroxyapatite probably has little meaning in relation to particles of molecular size, since such small aggregates of calcium and phosphate ions are known to be very unstable. Fortunately, experiments on the solubility of hydroxyapatite have shown that the simplest of all possible solubility products, namely 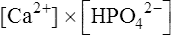 , best describes the actual solubility behaviour of this substance. The solubility products of hydroxyapatite itself, biological apatite and calcium hydrogen phosphate at pH 7·4, an ionic strength of 0·16 and 37°C (i.e. under physiological conditions) may therefore be considered as simple products of the calcium ion concentration and the concentration of the monohydrogen phosphate ion
, best describes the actual solubility behaviour of this substance. The solubility products of hydroxyapatite itself, biological apatite and calcium hydrogen phosphate at pH 7·4, an ionic strength of 0·16 and 37°C (i.e. under physiological conditions) may therefore be considered as simple products of the calcium ion concentration and the concentration of the monohydrogen phosphate ion 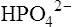 , which is the most abundant phosphate ion under these conditions.
, which is the most abundant phosphate ion under these conditions.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


