Chapter 8
Study Appraisal: Randomised Controlled Trials
Aim
The aim of this chapter is to outline the key elements of randomised controlled trials (RCTs) together with how to appraise an RCT, introduce the CONSORT statement and how to calculate simple measures of effect.
Outcomes
On completion of this chapter readers will understand the key features of an RCT and have 10 questions with which to appraise an RCT. They will also be able to calculate simple measures of effect.
What is an RCT?
The RCT is one of the simplest, most powerful tools of research. In essence, the RCT is a study in which people are allocated at random to receive one of several clinical interventions. In its simplest form it can be used to compare two treatments (see Fig 8-1).
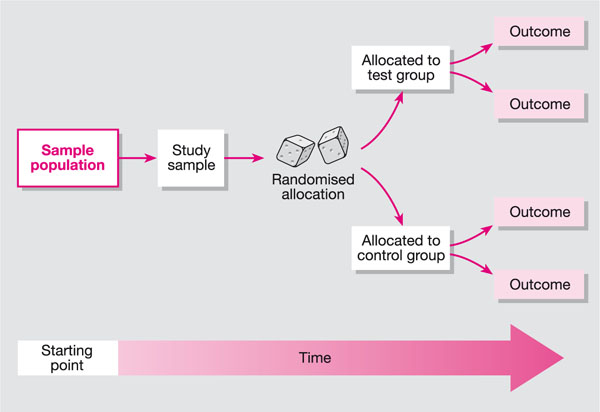
Fig 8-1 Randomised controlled trials.
Randomised controlled trials are the most rigorous way of determining whether a cause-and-effect relationship exists between treatment and outcome and for assessing the cost-effectiveness of a treatment.
RCTs are the best study design to answer questions related to therapy, prevention, aetiology and harm. Key elements of good RCTs are:
-
Good randomisation procedures (allocation concealment).
-
Patients masked (blinded) to whether they receive the test treatment or the control treatment.
-
Clinicians masked (blinded) to whether their patients receive the test treatment or the control treatment.
-
All participants followed up to the end of the study.
-
All participants analysed in the groups to which they were randomised (intention to treat).
The majority of tools available to appraise RCTs focus on helping the appraiser determine whether the key elements of RCTs mentioned above are present. A good example of one of these tools is available from the CASP website. The questions in the tool are derived from papers by Guyatt et al. (1993, 1994). See Fig 8-2.
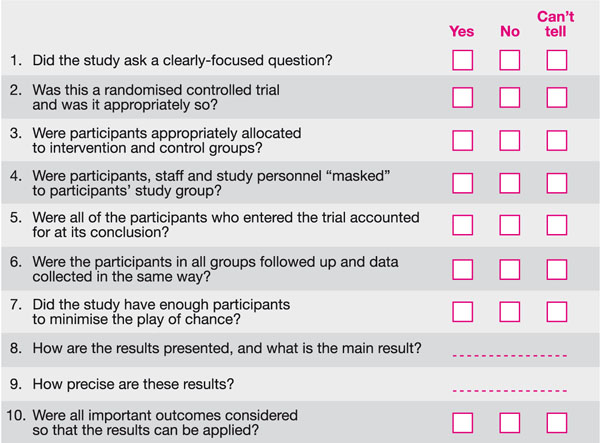
Fig 8-2 CASP 10 questions to appraise a randomised controlled trial.
Is the Study Clearly Focused?
This question is to help clarify whether the researchers have developed a clear research question. The process of developing a research question should be similar to the process you have been through to help develop your clear clinical questions. Consequently the study could have been investigating a clearly defined population (e.g. adults with rapidly progressing periodontal disease), comparing specific treatment (e.g. root planing and antimicrobial treatment versus root planing alone) or looking at clear outcomes. These outcomes may be clinical outcomes such as pocket depth, bleeding on probing or tooth or attachment loss, or patient-relevant outcomes such as pain, discomfort, or quality-of-life measures.
Is it a Randomised Controlled Trial?
You need to be clear that the trial was randomised and that a randomised controlled trial was the appropriate study design to answer the question.
The first two questions are designed to be screening questions. If you answer “no” to these first two questions there is likely to be little gained from reading the paper.
The remaining questions address the key elements of RCTs in more detail.
Were Participants Appropriately Allocated?
Randomisation is the only way to control for confounders that are not known or not measured in the study; therefore we need to know if the process of allocation of participants to the intervention and control groups was properly random. The best way of achieving this is to ensure the process is conducted by someone who is not recruiting patients, for example a central office. Good methods of randomisation are computer-generated random number sequences or random number tables.
In some cases, for example in a periodontal study, you may wish to separate out smokers and non-smokers before randomisation (stratification), to ensure a balance of smokers and non-smokers in the intervention and control groups.
With robust randomisation procedures you would expect there to be a balance of characteristics between the groups at baseline and for these to be discussed in the paper. You should consider whether any baseline differences would affect the outcome.
Were Participants, Staff and Study Personnel “Masked” to Participants’ Study Group?
If you are involved in testing a new material or treatment you are likely to have an opinion on whether or not it works. In some cases those conducting the study will have been involved in developing the material or treatment. Whatever role you may or may not have had, however balanced you think that you are, you will have a bias as to whether or not you think the material/treatment will work.
Consequently it is important that as many as possible of those involved in the trial are masked. This is not always possible; for example, two filling materials under test may be obviously different in colour. Being able to mask the individual assessing the outcome is key, if possible, as is being able to mask the patients.
Were All of the Participants who Entered the Trial Accounted for at its Conclusion?
In an ideal world all the patients who entered the trial at the beginning would complete the trial, all those assigned to the intervention under study would have received it, and all those in the control group would have received that option. Unfortunately things in real life are never that simple. It is not unusual for people to drop out of studies and on occasion for some to get the control option when they have been allocated to the intervention option and vice versa.
When participants drop out of a trial this is referred to as loss to follow-up. Loss to follow-up can have a significant effect on the study and investigators usually make strenuous efforts to ensure this is minimised. From an appraisal perspective it is important to know how many drop out in each group as this can have an important bearing on the result. If the authors have followed the CONSORT (Consolidated Standards of Reporting Trials) recommendations, which offer a standard method for the reporting of trials, these will be detailed in a flow chart (see Fig 8-3). More information about CONSORT can be found at www.consort-statement.org/ index.html.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


