Recurrent aphthous stomatitis (RAS) is the most common ulcerative disease affecting the oral mucosa. RAS occurs mostly in healthy individuals and has an atypical clinical presentation in immunocompromised individuals. The etiology of RAS is still unknown, but several local, systemic, immunologic, genetic, allergic, nutritional, and microbial factors, as well as immunosuppressive drugs, have been proposed as causative agents. Clinical management of RAS using topical and systemic therapies is based on severity of symptoms and the frequency, size, and number of lesions. The goals of therapy are to decrease pain and ulcer size, promote healing, and decrease the frequency of recurrence.
Key points
- •
Recurrent aphthous stomatitis (RAS) is the most common ulcerative disease of the oral mucosa.
- •
Several proposed etiologic theories are reviewed.
- •
Topical and systemic therapies that are used to manage RAS are presented.
Introduction
Recurrent aphthous stomatitis (RAS) remains the most common ulcerative disease of the oral mucosa, presenting as painful round, shallow ulcers with well-defined erythematous margin and yellowish-gray pseudomembranous center. RAS has a characteristic prodromal burning sensation that lasts from 2 to 48 hours before an ulcer appears. It occurs in otherwise healthy individuals and is typically located on the buccal and labial mucosa and tongue. Involvement of the heavily keratinized mucosa of the palate and gingiva is less common.
Diseases also causing oral ulcers that may be mistaken for RAS include Behçet disease, cyclic neutropenia, recurring intraoral herpes infections, human immunodeficiency virus (HIV)-related oral ulcers, or gastrointestinal diseases such as Crohn disease and ulcerative colitis. It is incumbent on the clinician managing oral disease to distinguish localized RAS from ulcers caused by an underlying systemic disorder.
Several factors have been proposed as possible causative agents for RAS, including: local factors, such as trauma in individuals who are genetically susceptible to RAS; microbial factors; nutritional factors, such as deficiency of folate and B-complex vitamins; immunologic factors; psychosocial stress; and allergy to dietary constituents. Extensive research has focused predominantly on immunologic factors, but a definitive etiology of RAS has yet to be clearly established.
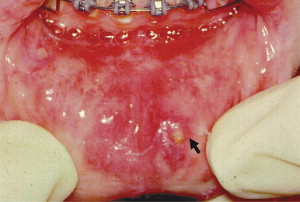
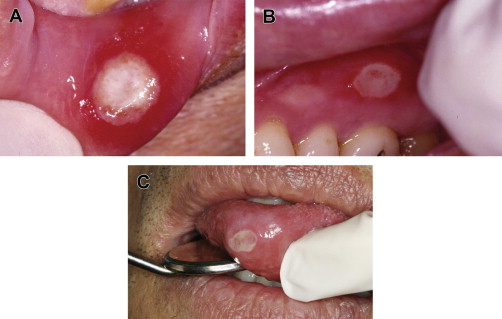
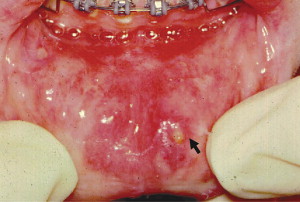
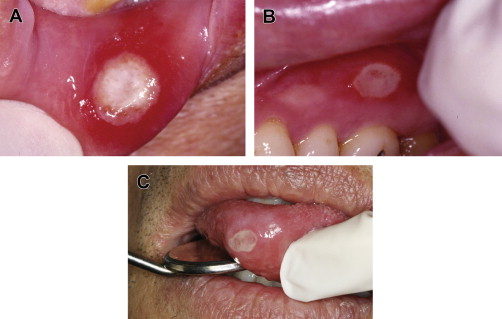
RAS is classified into minor, major, and herpetiform ulcers. More than 85% of RAS presents as minor ulcers that are less than 1 cm in diameter and heal without scars ( Fig. 1 ). Ulcers classified as major RAS, also known as Sutton disease or periadenitis mucosa necrotica recurrens, are larger than 1 cm in diameter, persist for weeks to months, and heal with scars ( Fig. 2 ). Herpetiform ulcers are clinically distinct because they appear as clusters of multiple ulcers scattered throughout the oral mucosa; despite the name, these lesions have no association with herpes simplex virus. General characteristics of the 3 types of RAS are summarized in Table 1 .
| Minor | Major | Herpetiform | |
|---|---|---|---|
| Gender predilection | M = F | M = F | F > M (usually) |
| Age at onset (y) | 5–19 | 10–19 | 20–29 |
| Number of ulcers | 1–5 | 1–10 | 10–100 |
| Size of ulcers (mm) | <10 | >10 | 1–2 (larger if coalesced) |
| Duration (d) | 4–14 | >30 | <30 |
| Recurrence rate (mo) | 1–4 | <1 | <1 |
| Site predilection | Lips, cheeks, tongue, floor of mouth | Lips, cheeks, tongue, palate, pharynx | Lips, cheeks, tongue, pharynx, palate, gingiva, floor of mouth |
| Permanent scarring | Unusual | Common | Unusual |
Management of RAS depends on the frequency and severity of the lesions. Most cases can be adequately managed with topical therapy, but systemic therapy is sometimes indicated for patients with major RAS or for those who experience large numbers of minor lesions that are nonresponsive to topical therapies.
Introduction
Recurrent aphthous stomatitis (RAS) remains the most common ulcerative disease of the oral mucosa, presenting as painful round, shallow ulcers with well-defined erythematous margin and yellowish-gray pseudomembranous center. RAS has a characteristic prodromal burning sensation that lasts from 2 to 48 hours before an ulcer appears. It occurs in otherwise healthy individuals and is typically located on the buccal and labial mucosa and tongue. Involvement of the heavily keratinized mucosa of the palate and gingiva is less common.
Diseases also causing oral ulcers that may be mistaken for RAS include Behçet disease, cyclic neutropenia, recurring intraoral herpes infections, human immunodeficiency virus (HIV)-related oral ulcers, or gastrointestinal diseases such as Crohn disease and ulcerative colitis. It is incumbent on the clinician managing oral disease to distinguish localized RAS from ulcers caused by an underlying systemic disorder.
Several factors have been proposed as possible causative agents for RAS, including: local factors, such as trauma in individuals who are genetically susceptible to RAS; microbial factors; nutritional factors, such as deficiency of folate and B-complex vitamins; immunologic factors; psychosocial stress; and allergy to dietary constituents. Extensive research has focused predominantly on immunologic factors, but a definitive etiology of RAS has yet to be clearly established.
RAS is classified into minor, major, and herpetiform ulcers. More than 85% of RAS presents as minor ulcers that are less than 1 cm in diameter and heal without scars ( Fig. 1 ). Ulcers classified as major RAS, also known as Sutton disease or periadenitis mucosa necrotica recurrens, are larger than 1 cm in diameter, persist for weeks to months, and heal with scars ( Fig. 2 ). Herpetiform ulcers are clinically distinct because they appear as clusters of multiple ulcers scattered throughout the oral mucosa; despite the name, these lesions have no association with herpes simplex virus. General characteristics of the 3 types of RAS are summarized in Table 1 .
| Minor | Major | Herpetiform | |
|---|---|---|---|
| Gender predilection | M = F | M = F | F > M (usually) |
| Age at onset (y) | 5–19 | 10–19 | 20–29 |
| Number of ulcers | 1–5 | 1–10 | 10–100 |
| Size of ulcers (mm) | <10 | >10 | 1–2 (larger if coalesced) |
| Duration (d) | 4–14 | >30 | <30 |
| Recurrence rate (mo) | 1–4 | <1 | <1 |
| Site predilection | Lips, cheeks, tongue, floor of mouth | Lips, cheeks, tongue, palate, pharynx | Lips, cheeks, tongue, pharynx, palate, gingiva, floor of mouth |
| Permanent scarring | Unusual | Common | Unusual |
Management of RAS depends on the frequency and severity of the lesions. Most cases can be adequately managed with topical therapy, but systemic therapy is sometimes indicated for patients with major RAS or for those who experience large numbers of minor lesions that are nonresponsive to topical therapies.
Epidemiology
Approximately 20% of the general population is affected by RAS, but incidence varies from 5% to 50% depending on the ethnic and socioeconomic groups studied. The prevalence of RAS is influenced by the population studied, diagnostic criteria, and environmental factors. In children, prevalence of RAS may be as high as 39%, and is influenced by the presence of RAS in one or both parents. Children with RAS-positive parents have a 90% chance of developing RAS, compared with 20% of those with RAS-negative parents. In children of high socioeconomic status, RAS is 5 times more prevalent and represents 50% of oral mucosal lesions in this cohort. RAS prevalence was found to be higher (male, 48.3%; female, 57.2%) among professional school students than in the same subjects 12 years later when they had become practicing professionals. This finding led some investigators to theorize that stress during student life is a major factor in RAS, although the differences attributable to age changes should also be considered. The onset of RAS appears to peak between the ages of 10 and 19 years and becomes less frequent with advancing age, geographic location, or gender. If RAS begins or significantly increases in severity after the third decade and well into adult life (see Table 1 ), it should increase suspicion that the cause of the condition may be attributed to an underlying medical disorder such as hematologic or immunologic abnormality, connective tissue disease, or Behçet syndrome.
Predisposing etiologic factors
The etiology of RAS lesions is still unknown, but several local, systemic, immunologic, genetic, allergic, nutritional, and microbial factors have been proposed as causative agents. Moreover, some medications including immunosuppressive drugs such calcineurin and mammalian target of rapamycin (mTOR) inhibitors have been associated with severe aphthous-like stomatitis ( Table 2 ).
| Local | Trauma Smoking Dysregulated saliva composition |
| Microbial | Bacterial: streptococci Viral: varicella zoster, cytomegalovirus |
| Systemic | Behçet disease Mouth and genital ulcers with inflamed cartilage (MAGIC) syndrome Crohn disease Ulcerative colitis Human immunodeficiency virus infection Periodic fever, aphthosis, pharyngitis, and adenitis (PFAPA) or Marshall syndrome Cyclic neutropenia Stress; psychological imbalance, menstrual cycle |
| Nutritional | Gluten-sensitive enteropathy Iron, folic acid, zinc deficiencies Vitamin B 1 , B 2 , B 6 , and B 12 deficiencies |
| Genetic | Ethnicity Human leukocyte antigen haplotypes |
| Allergic/immunologic | Local T-lymphocyte cytotoxicity Abnormal CD4:CD8 ratio Dysregulated cytokine levels Microbe-induced hypersensitivity Sodium lauryl sulfate sensitivity Food sensitivity |
| Others | Antioxidants Nonsteroidal anti-inflammatory drugs β-Blockers Immunosuppressive drugs |
Local Factors
Local trauma is regarded as a causative agent for RAS in susceptible individuals. Trauma predisposes to RAS by inducing edema and early cellular inflammation associated with an increased viscosity of the oral submucosal extracellular matrix. Not all oral trauma leads to RAS, because denture wearers do not have a high prevalence of RAS despite this cohort being 3 times more susceptible to oral mucosal ulceration. In addition, habitual smokers who constantly expose their oral mucosa to nicotine have demonstrated a negative association between smoking and RAS. Therefore, local trauma apparently predisposes to RAS only in those individuals who have a hereditary predilection for the disease.
Some changes in salivary composition that affect the local properties of saliva, such as pH and a stress-induced increase in salivary cortisol, have been correlated with RAS. Although direct association of salivary gland dysfunction with RAS has not been demonstrated, patients with a combination of RAS and xerostomia may experience increased symptoms resulting from the increased oral dryness.
Microbial Factors
Despite RAS having not been etiologically associated with herpes simplex virus based on several well-designed studies, both laymen and clinicians often confuse RAS with herpes simplex virus (HSV) infection. HSV virions and antigens have neither been identified in aphthous lesions nor successfully isolated in RAS biopsy tissues. Although it has been suggested that reactivation of varicella zoster virus (VZV) or human cytomegalovirus (CMV) is associated with frequent recurrence of aphthous ulcers, evaluation of RAS biopsy tissue using polymerase chain reaction (PCR) for possible involvement of herpes virus 6, CMV, VZV, or Epstein-Barr virus (EBV) as causative factors did not find evidence to support the role of these viruses in RAS pathogenesis. Thus, it is the clinician’s responsibility to distinguish RAS from herpes infections and to reassure RAS patients that they do not have an infectious disease, and that antiviral therapy is neither necessary nor effective.
Helicobacter pylori , a common risk factor for gastric and duodenal ulcers, has been proposed to have a causative role in RAS. Despite the fact that stomach ulcers and RAS are linked to dysregulated immune functions, molecular studies that identified H pylori in both affected and nonaffected mucosa of RAS patients found no association with RAS. Of note, another study reported that eradication of H pylori in RAS patients positively correlated with increased Vitamin B 12 levels and decreased the number of aphthous lesions. There has been considerable speculation regarding the possible involvement of Streptococcus species in the etiology of RAS, especially Streptococcus sanguis 2A. The proposed hypothesis is that oral streptococci act as antigenic stimulants that cross-react with mitochondrial heat-shock proteins of oral keratinocytes. This reaction purportedly induces a T-cell–mediated immune response that causes oral mucosal damage, but this theory remains unproven. EBV and Lactobacillus are other organisms that have been studied in RAS patients. A study of the possible role of Lactobacillus in RAS has yielded no significant finding; but in a small study sample, EBV was associated with epithelial cells of preulcerative RAS. Using PCR techniques, 39% of preulcerative RAS lesions were positive for EBV DNA. Their peripheral blood lymphocytes and serum were also positive for EBV DNA. The report theorized that lymphocytes may serve as a reservoir for latent EBV infection and promote viral shedding into the plasma. However, a causal relationship between Epstein-Barr viral load and RAS was not evaluated.
Underlying Disease
The most prominent medical disorder associated with RAS is Behçet syndrome, characterized by recurring oral and genital ulcers, and eye lesions (see Table 2 ). Behçet syndrome is a multisystem disorder resulting from vasculitis of small and medium-sized vessels and inflammation of epithelium. The abnormal inflammatory response in Behçet syndrome is caused by immune complexes induced by T lymphocytes and plasma cells. Although Behçet syndrome usually affects adults, several cases have been reported in children.
Because distinguishing RAS from Behçet disease now depends on clinical criteria, investigators have sought an effective laboratory test. A high titer of anti– Saccharomyces cerevisiae antibodies (ASCA) has been detected in Behçet patients relative to RAS patients and apparently healthy individuals. This report suggested that the ASCA test might be a method to distinguish between these 2 patient populations. This distinction may not be as simple as reported, because up to 70% of patients with Crohn disease and 15% of patients with ulcerative colitis are ASCA positive, and both diseases are associated with recurring oral ulcers. The use of the human leukocyte antigen (HLA) system to distinguish RAS from Behçet disease showed significant differences in the frequency of certain HLA antigens, but the distinction between the 2 disorders is still not clearly defined.
Another variant of Behçet syndrome that includes relapsing polychondritis, a disorder characterized by mouth and genital ulcers with inflamed cartilage, has been labeled MAGIC syndrome (see Table 2 ).
Inflammatory bowel diseases such as Crohn disease and ulcerative colitis have been associated with oral ulcers that may resemble RAS, but Crohn lesions often have indurated borders and are histologically different because of the granulomatous nature of the lesion ( Fig. 3 ). Approximately 10% of patients with Crohn disease have oral mucosal ulcers, and the oral manifestations occasionally precede intestinal symptoms. Some researchers believe that inflammation of minor salivary glands is a possible cause of the oral ulcers.
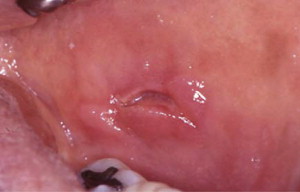
Celiac disease, an autoimmune sensitivity to gluten, is another medical disorder often associated with RAS, but the causal relationship between these 2 disorders is not completely clear. Prevalence of RAS in patients with celiac disease has been reported to range from 4% to 40%, but oral ulceration in such patients also varies from 3% to 61%. In addition, oral ulcerations in celiac disease do not have the distinctive features of RAS, and often resolve when celiac disease patients are placed on gluten-free diet. Therefore, oral ulceration in patients with celiac disease may not be the typical RAS.
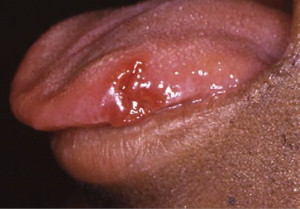
In HIV-positive individuals, RAS occurs more frequently, lasts longer, and causes more painful symptoms than in healthy individuals ( Fig. 4 ). It is also a common finding in HIV-positive children. RAS is usually a late finding in AIDS patients with CD4 + lymphocyte counts of fewer than 100 cells/mm 3 , and it may occasionally be a presenting sign of HIV infection.
Cyclic neutropenia, a rare disorder that presents in childhood, is also associated with recurring oral ulcers during periods when the neutrophil count is severely depressed. Another condition, described as periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) or Marshall syndrome, has a presentation similar to that of cyclic neutropenia and is commonly associated with oral ulcers that cannot be distinguished from RAS.
Hereditary and Genetic Factors
The role of heredity is the best-defined underlying cause of RAS. Susceptibility to RAS is significantly increased by its presence in one or both parents. Studies of identical twins have also demonstrated the hereditary nature of this disorder. Individuals with a positive family history of RAS tend to develop RAS at an early age. Specifically, children with two RAS-positive parents have a 90% chance of developing RAS that presents with more severe symptoms and recurs more frequently.
Certain genetically specific HLAs have been identified in RAS patients: HLA-A2, HLA-B5, HLA-B12, HLA-B44, HLA-B51, HLA-B52, HLA-DR2, HLA-DR7, and HLA-DQ series. A confounding finding is that certain ethnic groups have been associated with different HLA alleles or haplotypes, with no HLA being consistently associated with RAS. Additional studies are therefore needed to clarify the variability of RAS in host susceptibility.
Allergic Factors
Allergy has been suspected as a cause of RAS. Hypersensitivity to certain food substances, oral microbes such as S sanguis , and microbial heat-shock protein have been suggested as possible causative factors, but there is still no conclusive evidence to support allergy as a major cause of RAS. Although some studies reported that RAS patients tend to have hypersensitivity to environmental allergens, other reports did not find a significant correlation between hypersensitivity and RAS. In one report, patients wearing nickel-based orthodontic appliances developed RAS that coincided with fitting of the appliance. When the appliance was replaced with a nickel-free type, the mucosal lesion regressed. RAS in this population was attributed to the systemic effect of ingested nickel rather than direct contact, because a patch test to nickel sulfate did not reactivate the mucosal ulceration. In patients presenting with refractory cases of RAS and known allergy to food items such as milk, cheese, and wheat, sequential elimination of these dietary items was found to be beneficial in a small subset of RAS patients, thereby suggesting a possible link between food allergy and some cases of RAS.
The denaturing effect of sodium lauryl sulfate (SLS), commonly found in toothpastes, has also been discussed as a cause of RAS. It was proposed that SLS might erode the oral mucin layer, exposing the underlying epithelium, thereby making the individual more susceptible to RAS. This theory still needs further clarification, because it has also been demonstrated that the use of SLS-free toothpastes did not affect the development of new lesions in RAS patients.
Immunologic Factors
There has been significant research on the cause of RAS, focusing on detecting an abnormality in the immunologic response. Early work suggested a relationship between several immune-mediated reactions and the development of RAS. These reactions include cytotoxicity of T lymphocytes to oral epithelium, antibody-dependent cell-mediated cytotoxicity, and defects in lymphocyte subpopulations. One theory is that multiple immune reactions cause damage induced by deposition of immune complexes within the oral epithelium. In some patients, an elevated level of salivary immunoglobulin A has been reported during acute and remission phases of minor RAS. Some other studies have shown an association between RAS severity and abnormal proportions of CD4 + and CD8 + cells, alteration of the CD4 + :CD8 + ratio, and increased levels of several cytokines including interleukin-2, interferon-γ, and tumor necrosis factor-α (TNF-α) mRNA in RAS lesions. Immunohistochemical studies of RAS biopsy tissues demonstrated numerous inflammatory cells with variable ratios of CD4 + :CD8 + T lymphocytes depending on the ulcer duration. CD4 + cells were more numerous during the preulcerative and healing stages, whereas CD8 + cells tended to be more numerous during the ulcerative state of the ulcer. It is interesting that studies on nonaffected sites were negative, making researchers focus more on the theory that RAS may be caused by an antigen-triggering effect. Because levels of serum immunoglobulins and natural killer cells are essentially within normal limits in RAS patients, the focus is still on a dysregulated local cell-mediated immune response conducive to accumulation of subsets of T cells, mostly CD8 + cells. This local immune response is thought to cause tissue breakdown that eventually manifests as RAS.
Nutritional Factors
The role of nutritional deficiency as a cause of RAS has been highlighted by the association of a subset of 5% to 10% of RAS patients with low serum levels of iron, folate, zinc, or vitamins B 1 , B 2 , B 6 , and B 12 , which indicates that nutritional deficiency is an etiologic factor for RAS. Some of these nutritional deficiencies may be secondary to other diseases such as malabsorption syndrome or gluten sensitivity associated with (or without) enteropathy. Hematologic screening of RAS patients for anemia or deficiency of iron, folate, and B vitamins is appropriate for patients with major RAS or cases of minor RAS that worsen during adult life. A deficiency of calcium and vitamin C has also been proposed in patients with RAS, but these findings were in association with vitamin B 1 deficiency, supporting the idea of combined nutritional deficiency in RAS patients. The recovery of some RAS patients after treatment of the nutritional deficiency has further corroborated the causative role of nutritional deficiency in a subset of RAS patients.
Psychological Stress
Stress and psychological imbalance have been associated with RAS. Stressful life events can increase the chances that a RAS-susceptible patient will develop a new lesion. One study reported that mental stress is more strongly associated with episodes of RAS than is physical stress, and that these stressful events tend to correlate more with onset of RAS than with duration of the lesions. In women, appearance of RAS may coincide with menses. Stress of academic load may be the precipitating factor for the higher prevalence of RAS in professional school students. Clinicians should consider questioning patients with worsening episodes of RAS regarding psychosocial, physical, or environmental stress.
Other Factors
The role of antioxidants in RAS is still attracting attention because blood and salivary levels of antioxidants, such as erythrocyte superoxide dismutase and catalase, seem to be higher in patients with RAS and Behçet syndrome than in normal controls, but their causative roles in RAS are yet to be clearly defined. There have also been several reported cases of drug-induced RAS. A case-control study associated a higher risk of RAS with drug exposure, and found a significant association with nonsteroidal anti-inflammatory drugs and β-blockers. Nicorandil, a vasodilator used extensively outside the United States to manage angina, as well as calcineurin and mTOR inhibitors used as immunosuppressors, have been associated with severe aphthous-like ulcers. Therefore, it is imperative to closely scrutinize the medication history and current medications of RAS patients to identify any pattern associated with the frequency and duration of RAS lesions.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


