History of the Procedure
The relationship of the parathyroid gland and hyperparathyroidism was first recognized in 1903 by Askanazy, and the theory that parathyroid adenoma may be responsible for hyperparathyroidism was put forth by Schlagenhaufer in 1915. The first operation to remove a parathyroid tumor was performed by Felix Mandl in Vienna, Austria, in 1925 with local anesthesia. Subsequent work by names such as Albright, Castleman, and Cope set the stage for our current understanding of the pathology of parathyroid glands and the surgical management of hyperparathyroidism. In the 1950s and 1960s, an understanding developed of the activity of parathyroid hormone. Continued understanding of the anatomy of the parathyroid region and gland physiology led to improved gland localization and improved accuracy of surgical technique. The introduction of intraoperative parathyroid hormone (PTH) monitoring was revolutionary in the management of benign parathyroid disease. However, despite more than 100 years of parathyroid surgery, the historical challenges—including gland localization, determining biologic cure, and reoperative surgery—remain relevant today.
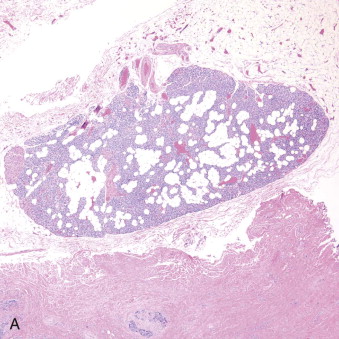
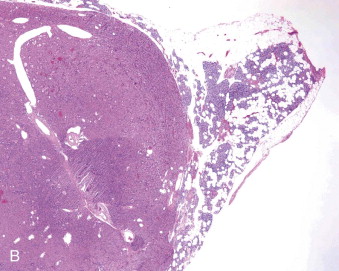
Hyperparathyroidism is a disorder of bone and mineral metabolism caused by the hypersecretion of the PTH. The majority of cases (80% to 90%) are due to solitary secretory parathyroid adenoma ( Figure 90-1 ). This is termed primary hyperthyroidism, the subject of this chapter. The prevalence of primary hyperparathyroidism is estimated to be 0.2% to 1%. Between 5% and 15% of cases of primary parathyroid hyperplasia involve four-gland hyperplasia. Secondary and tertiary hyperparathyroidism are related to renal disease or other disruptions of calcium metabolism. Secondary hyperparathyroidism is a result of overactive parathyroid glands in the setting of renal osteodystrophy. Tertiary hyperparathyroidism typically occurs in patients with longstanding secondary hyperparathyroidism who undergo renal transplantation. Parathyroid carcinoma, although rare, may cause significant hyperparathyroidism and can be associated with debilitating symptoms. These types of hyperparathyroidism are beyond the scope of this chapter, although surgical treatment is often indicated in these disorders as well.
Surgical intervention is the mainstay for the treatment of primary hyperparathyroidism. Traditionally, resection of the abnormal parathyroid glands was accomplished by a bilateral exploration with evaluation of all four parathyroid glands. Intraoperative frozen section confirmation of parathyroid tissue was performed, and the offending gland was then resected. Current strategies have progressed toward a more minimally invasive approach with guided parathyroid gland surgery and removal of the offending gland as identified preoperatively. Surgical techniques vary widely and include open approaches, radioguided parathyroidectomy, video-assisted parathyroidectomy, as well as endoscopic parathyroidectomy. Currently, 10% of surgeons practice bilateral neck exploration, 68% practice limited exploration, and 22% have a mixed practice. Most surgeons prefer a focal, single-gland examination under general anesthesia and 23-hour observation. Interestingly, controversy related to long-term outcomes still exists among surgeons regarding routine bilateral exploration versus minimally invasive approaches, despite advances in biochemical testing, imaging, and surgical techniques since the 1960s.
Preoperative Parathyroid Localization
One of the most important steps in the preoperative evaluation of a patient with hyperparathyroidism, when primary hyperparathyroidism is suspected, is localization of the adenoma. Various imaging modalities are utilized to pinpoint the offending parathyroid gland or glands. These include ultrasound, 99mTc-sestamibi, computed tomography (CT), magnetic resonance imaging (MRI), and a variety of other imaging modalities. Most surgeons employ a combination of 99mTc-sestamibi and cervical ultrasound of the parathyroid glands. Nuclear imaging scan uses a radiotracer that is preferentially taken up by the metabolically active parathyroid, thyroid, and cardiac tissue. The tracer washes out of the thyroid gland, revealing the location of the enlarged parathyroid glands. Because 99mTc-sestamibi scanning can be difficult to interpret and has issues with sensitivity, alternative imaging modalities are often utilized. Fusion with single-photon emission computed tomography (SPECT) or CT scanning improves anatomic information. Data suggest that the combination of CT-99mTc-sestamibi-SPECT is the best approach for preoperative localization for patients with one-gland disease. The addition of ultrasound is likely more sensitive than 99mTc-sestamibi, but this decreases when thyroid nodules are present. Deep superior glands and other ectopic glands are also difficult to localize via ultrasound. Also, parathyroid adenomas in the mediastinum are generally seen by 99mTc-sestamibi scans but not by ultrasound. Additional modalities such as CT protocols, MRI, positron emission tomography (PET), and 4D-CT offer excellent promise to improve the localization of adenomas in the future and vary depending on the institution. Four-dimensional CT may offer superior accuracy compared with that of ultrasonography and technetium 99m sestamibi scanning. MRI with contrast has superior soft tissue imaging capabilities relative to CT and may be helpful in identifying ectopic parathyroid glands. Sensitivity is reported to be greater than 75% for localization of adenomas. Positron emission tomography scanning may have a role in these situations as well.
History of the Procedure
The relationship of the parathyroid gland and hyperparathyroidism was first recognized in 1903 by Askanazy, and the theory that parathyroid adenoma may be responsible for hyperparathyroidism was put forth by Schlagenhaufer in 1915. The first operation to remove a parathyroid tumor was performed by Felix Mandl in Vienna, Austria, in 1925 with local anesthesia. Subsequent work by names such as Albright, Castleman, and Cope set the stage for our current understanding of the pathology of parathyroid glands and the surgical management of hyperparathyroidism. In the 1950s and 1960s, an understanding developed of the activity of parathyroid hormone. Continued understanding of the anatomy of the parathyroid region and gland physiology led to improved gland localization and improved accuracy of surgical technique. The introduction of intraoperative parathyroid hormone (PTH) monitoring was revolutionary in the management of benign parathyroid disease. However, despite more than 100 years of parathyroid surgery, the historical challenges—including gland localization, determining biologic cure, and reoperative surgery—remain relevant today.
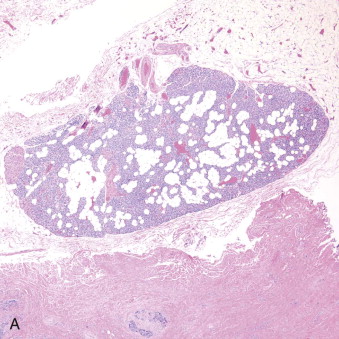
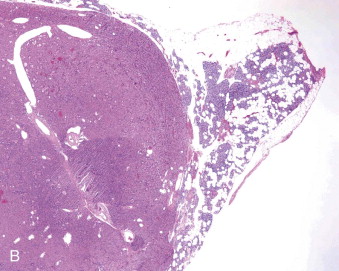
Hyperparathyroidism is a disorder of bone and mineral metabolism caused by the hypersecretion of the PTH. The majority of cases (80% to 90%) are due to solitary secretory parathyroid adenoma ( Figure 90-1 ). This is termed primary hyperthyroidism, the subject of this chapter. The prevalence of primary hyperparathyroidism is estimated to be 0.2% to 1%. Between 5% and 15% of cases of primary parathyroid hyperplasia involve four-gland hyperplasia. Secondary and tertiary hyperparathyroidism are related to renal disease or other disruptions of calcium metabolism. Secondary hyperparathyroidism is a result of overactive parathyroid glands in the setting of renal osteodystrophy. Tertiary hyperparathyroidism typically occurs in patients with longstanding secondary hyperparathyroidism who undergo renal transplantation. Parathyroid carcinoma, although rare, may cause significant hyperparathyroidism and can be associated with debilitating symptoms. These types of hyperparathyroidism are beyond the scope of this chapter, although surgical treatment is often indicated in these disorders as well.
Surgical intervention is the mainstay for the treatment of primary hyperparathyroidism. Traditionally, resection of the abnormal parathyroid glands was accomplished by a bilateral exploration with evaluation of all four parathyroid glands. Intraoperative frozen section confirmation of parathyroid tissue was performed, and the offending gland was then resected. Current strategies have progressed toward a more minimally invasive approach with guided parathyroid gland surgery and removal of the offending gland as identified preoperatively. Surgical techniques vary widely and include open approaches, radioguided parathyroidectomy, video-assisted parathyroidectomy, as well as endoscopic parathyroidectomy. Currently, 10% of surgeons practice bilateral neck exploration, 68% practice limited exploration, and 22% have a mixed practice. Most surgeons prefer a focal, single-gland examination under general anesthesia and 23-hour observation. Interestingly, controversy related to long-term outcomes still exists among surgeons regarding routine bilateral exploration versus minimally invasive approaches, despite advances in biochemical testing, imaging, and surgical techniques since the 1960s.
Preoperative Parathyroid Localization
One of the most important steps in the preoperative evaluation of a patient with hyperparathyroidism, when primary hyperparathyroidism is suspected, is localization of the adenoma. Various imaging modalities are utilized to pinpoint the offending parathyroid gland or glands. These include ultrasound, 99mTc-sestamibi, computed tomography (CT), magnetic resonance imaging (MRI), and a variety of other imaging modalities. Most surgeons employ a combination of 99mTc-sestamibi and cervical ultrasound of the parathyroid glands. Nuclear imaging scan uses a radiotracer that is preferentially taken up by the metabolically active parathyroid, thyroid, and cardiac tissue. The tracer washes out of the thyroid gland, revealing the location of the enlarged parathyroid glands. Because 99mTc-sestamibi scanning can be difficult to interpret and has issues with sensitivity, alternative imaging modalities are often utilized. Fusion with single-photon emission computed tomography (SPECT) or CT scanning improves anatomic information. Data suggest that the combination of CT-99mTc-sestamibi-SPECT is the best approach for preoperative localization for patients with one-gland disease. The addition of ultrasound is likely more sensitive than 99mTc-sestamibi, but this decreases when thyroid nodules are present. Deep superior glands and other ectopic glands are also difficult to localize via ultrasound. Also, parathyroid adenomas in the mediastinum are generally seen by 99mTc-sestamibi scans but not by ultrasound. Additional modalities such as CT protocols, MRI, positron emission tomography (PET), and 4D-CT offer excellent promise to improve the localization of adenomas in the future and vary depending on the institution. Four-dimensional CT may offer superior accuracy compared with that of ultrasonography and technetium 99m sestamibi scanning. MRI with contrast has superior soft tissue imaging capabilities relative to CT and may be helpful in identifying ectopic parathyroid glands. Sensitivity is reported to be greater than 75% for localization of adenomas. Positron emission tomography scanning may have a role in these situations as well.
Indications for the Use of the Procedure
With the use of routine blood screening tests, most patients who present with hyperparathyroidism are asymptomatic. Many patients express somewhat nonspecific symptoms such as fatigue, musculoskeletal pains and aches, depression, constipation and abdominal discomfort, and decreased memory. Symptoms related to the effects of PTH may occur, however, and some patients may present with renal calculi (10% to 20%) or rarely osteitis fibrosis. Current guidelines for the indications for parathyroidectomy are as follows:
- •
Serum calcium greater than 1 mg/dL (0.25 mmol/L) above normal levels (generally >12 mg/dL)
- •
Creatinine clearance reduced to less than 60 mL/min
- •
Overt manifestations of hyperparathyroidism (such as nephrolithiasis, osteitis fibrosa cystica, or classic neuromuscular disease)
- •
Bone density T score <2.5 (greater than 2.5 standard deviations below peak mass) standard deviation at any site or previous fragility fracture at the lumbar spine, hip, or distal radius
- •
Age <50 years
- •
Note that elevated 24-hour urine calcium is no longer an indication (previously >400 mg/day was a recommendation for parathyroidectomy)
Patients with an elevated intact PTH level and a calcium concentration in the normal range often have secondary hyperparathyroidism. Secondary hyperparathyroidism can result from insufficient calcium or vitamin D intake, decreased intestinal calcium absorption, vitamin D malabsorption, or renal hypercalciuria. Parathyroidectomy in the setting of secondary hyperparathyroidism related to renal failure is a complex surgical issue. Supernumerary parathyroid tissue in ectopic locations is commonly encountered (discussed later). Thorough preoperative workup and surgical exploration with excision of the offending glandular tissue will ensure the best outcomes and should be performed by experienced clinicians. Cervical thymectomy may also be indicated due to intrathymic parathyroid glands.
Limitations and Contraindications
Although there are several relative contraindications for parathyroidectomy depending on the clinical situation, an absolute contraindication is the presence of familial hypocalciuric hypercalcemia (FHH). Patients can present with elevated calcium and PTH levels, mimicking the serum biochemical characteristics of primary hyperparathyroidism. The differentiation of FHH from primary hyperparathyroidism may be revealed with the 24-hour urine calcium excretion. In FHH, the secretion of calcium is lower than expected in comparison with the serum calcium level. Calcium excretion over 24 hours is <100 mg in a majority of FHH patients but usually >200 mg in patients with primary hyperparathyroidism. In addition PTH levels are generally mildly elevated or normal in FHH. FHH is not treated with parathyroidectomy, and referral to an endocrinologist is warranted.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


