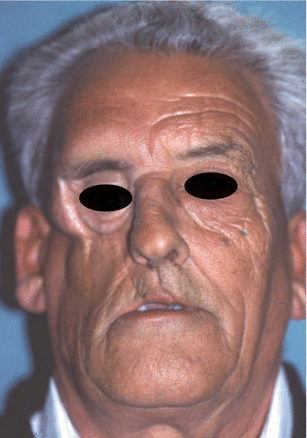
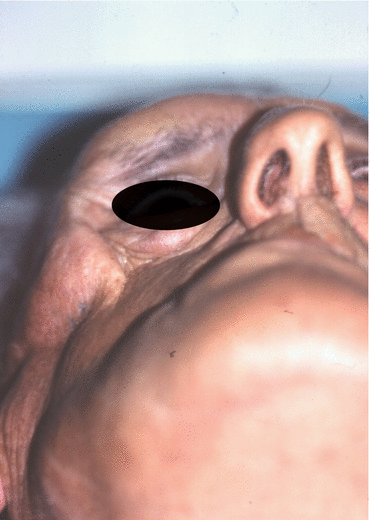
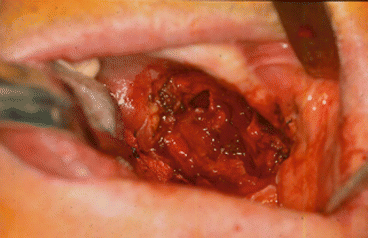
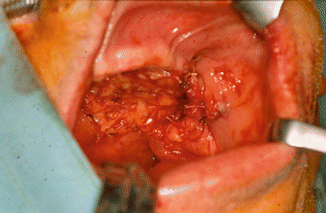
Fig. 2.1
Vertical and horizontal defects. Brown classification 2010
Poetker et al. [18] report complications arising from surgery, including cerebrospinal fluid leak, mucocele, trigeminal nerve pain, osteomyelitis, perforation of the nasal septum, oroantral and oronasal communications, loss of midface projection, nasal collapse, formation of synechiae, alar retraction, and ectropion.
2.3 Reconstructive Techniques
2.3.1 Obturators
An obturator is any dental prosthesis that prevents oroantral and oronasal communication while reestablishing dentition and speech. It is a simple, nonsurgical option. Pittman and Zender [19] favor performing a dental assessment before surgery so that the necessary extractions of infected teeth can be made and an impression can be taken for the obturator if reconstruction of resected tissue is not planned. However, it may be difficult for these prostheses to remain in place, as they are made before surgery. Park and Kwon [20] maintain that delayed surgical obturators can provide better functional results than immediate obturators.
We are against the use of obturators for the following disadvantages:
The nineteenth century saw the first descriptions of pedicle flaps, which were an alternative in the management of maxillectomy defects. Other surgical techniques became available over time, including nonvascularized grafts, free flaps, and distraction osteogenesis. The size of the soft or hard tissue defect is crucial when deciding on a reconstruction technique. Nevertheless, there is no consensus on the ideal approach for each type of defect [1].
2.3.2 Local Flaps
Local and regional flaps are the first choice for small defects such as those affecting the palate, maxillary tuberosity, and oroantral communications.
2.3.2.1 Bichat Fat Pad
This structure was reported by Bichat in 1802, although it had already been described by Heister in 1732 and Winslow in 1753. Egyedi was first to use it for reconstruction of the oral cavity in 1977 [21–25].
Vascularization depends on three arteries: the buccal branch and the deep temporal branch, both of which are branches of the internal maxillary artery, and the transverse facial artery, which is a branch of the superficial temporal artery. Blood supply is also provided by small branches of the facial artery. Such diversity of supply ensures the safety of this option.
2.3.3 Regional Pedicle Flaps
Regional pedicle flaps were first used in the nineteenth century. Flap survival always depends on integrity and appropriate vascularization once the flap has been surgically designed.
According to Muzaffar et al. [26], the first flaps used for reconstruction of the midface were the deltopectoral flap, pectoralis major flap, latissimus dorsi flap, temporalis flap, sternocleidomastoid flap, and trapezius flap. The pectoralis, latissimus dorsi, and trapezius are too bulky and difficult to adapt for most defects.
Pedicle flaps are used for larger defects. Carstens et al. [27] stated that pedicle flaps were limited by the length of the vascular pedicle and the lack of sufficient tissue to fill the defect. Their intrinsic characteristics make these flaps ideal for elderly patients with a high cardiovascular risk.
2.3.3.1 Buccinator Flap
The buccinator is made of flexible, thin tissue, and, according to Zhao et al. [28], its properties are satisfactory. It is covered medially by mucosa and laterally by the masseter, the ascending mandibular branch, the Bichat fat pad, and the buccopharyngeal fascia. Anteriorly, it is intertwined with the orbicularis oculi; posteriorly, it inserts into the pterygomandibular ligament.
Kaplan [29] first used the buccal mucosa in 1975, followed by Maeda et al. [30], who incorporated fibers from the buccinator. Bozola et al. [31] reported that the buccal artery and facial artery [27, 32] provided the main supply to the flap. Venous drainage is by the pterygoid plexus and the facial vein.
The buccinator can be used for posterior-based flaps. It can be tunneled below the pterygomandibular ligament to close defects of the palatine and pharyngeal mucosa, dental alveolus, and floor of the mouth. Its design can also be anterior based to close defects of the anterior hard palate, dental alveolus, maxillary sinus, floor of the nasal cavity, lip, and orbit.
Nowadays, we do not use this flap for any maxillary defects.
2.3.3.2 Temporalis Muscle Flap
The temporalis muscle flap was first described in 1898 by Golovine [33] for reconstruction of an orbital defect after exenteration.
In a study with rhesus monkeys in 1981, Bradley and Brockbank [34] reported the possibility of rotating the temporalis muscle toward the mouth after division of the zygomatic arch and base of the coronoid.
Cheung [35] described the rich microvascular network of the temporalis muscle, which depends on three main arteries: the anterior deep temporal artery, the posterior deep temporal artery, and the middle temporal artery. The first two arteries are branches of the internal maxillary artery, whereas the third arises from the superficial temporal artery. Such extensive vascularization makes the temporalis flap a very safe reconstructive technique for intraoral defects, with relatively few complications during recovery. Survival of this flap is based on preservation of the deep temporal artery.
Ahmed Djae et al. [36] report the following advantages for the temporalis muscle flap:
-
Both the flap and the area to be reconstructed are found in the same surgical field.
-
The muscle is quite bulky.
-
Adequate vascularization.
-
The flap comprises muscle and fascia.
-
Minimal morbidity.
-
The cosmetic defect in the temporal fossa can be corrected with a prosthesis.
We use this flap for Brown type IA and IIA defects [17]. In the case of type IIIA defects, we use it to reconstruct the palate in combination with other flaps.
Functional rehabilitation is done with a mixed prosthesis based on the contralateral teeth and a removable partial denture with attachments or implants in the contralateral maxilla. In our experience, we place Mozo Grau implants (MG osseous) in the contralateral maxilla with excellent results.
Although Cheung [35] describes submucosal neovascularization during the first weeks after muscle transfer, the approach does permit subsequent surgery for reconstruction with cortical-cancellous chips and titanium mesh before dental rehabilitation with implants. We do not use this technique.
Complications are often associated with the size of the defect and the location of the structure to be replaced. They include the following:
-
Oroantral communications
-
Partial/total flap loss
-
Difficulties with chewing and/or mouth opening
-
Paresis/paralysis of the frontal branch of the facial nerve
-
Hematoma, superinfection, and cosmetic defects at the donor site (Figs. 2.6, 2.7, 2.8, 2.9, 2.10, 2.11, and 2.12)
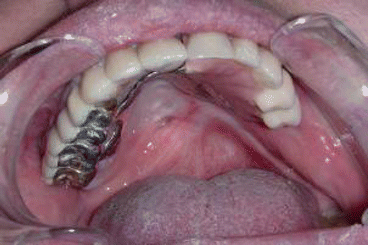 Fig. 2.6Palatal reconstruction after IA maxillectomy
Fig. 2.6Palatal reconstruction after IA maxillectomy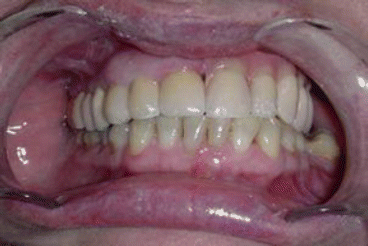 Fig 2.7Definitive occlusion
Fig 2.7Definitive occlusion Fig. 2.8Postoperative view
Fig. 2.8Postoperative view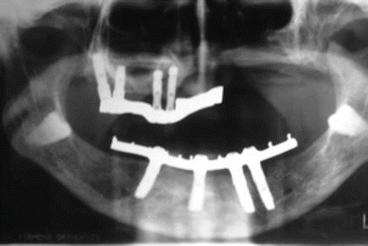 Fig. 2.9Prosthetic rehabilitation with dental implants on the contralateral hemimaxilla
Fig. 2.9Prosthetic rehabilitation with dental implants on the contralateral hemimaxilla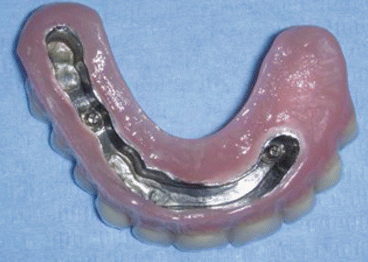 Fig. 2.10Prosthesis
Fig. 2.10Prosthesis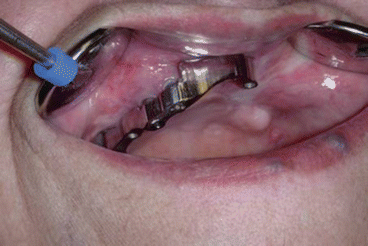 Fig. 2.11Postoperative intraoral view
Fig. 2.11Postoperative intraoral view Fig. 2.12Postoperative view
Fig. 2.12Postoperative view
2.3.3.3 Temporoparietal Fascial Flap with Parietal Bone Flap
In 1898, Brown [37] and Monks [38] first reported using this flap to reconstruct the external ear and upper eyelid.
The temporoparietal fascial flap has a very long axis of rotation, which, according to Roy et al. [39], enables high mobility. Furthermore, Lam and Carlson [40] report that the anatomy of the temporal artery system is very constant, with blood supply to the flap provided by the superficial temporal vein and artery.
Mokal et al. [41] state that this reconstruction technique can be jeopardized by local traumatic injuries, previous surgery, radiation therapy, and carotid artery occlusion.
Its advantages include the following:
-
Large area (approximately 14 × 17 cm)
-
Thinness (2–4 mm)
-
Inclusion of parietal bone
Its complications include the following:
-
Possible lesion of the frontal branch of the facial nerve
-
Unsightly scars
-
Alopecia on scar tissue
We rarely use this flap; sometimes we apply it to maxillectomy IIIA in combination with temporalis muscle flap. The parietal bone supports the eye in the right position, and the temporalis myofascial flap reconstructs the palate.
It can include parietal bone for reconstruction of malar bone defects.
Therefore, we use it in type IIA defects, where it holds the eyeball in place (Figs. 2.13, 2.14, 2.15, and 2.16).
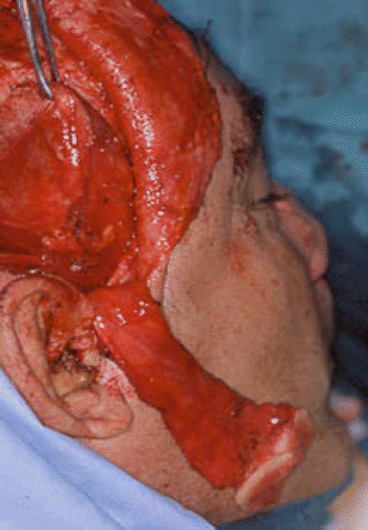
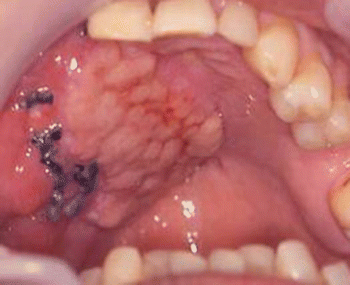
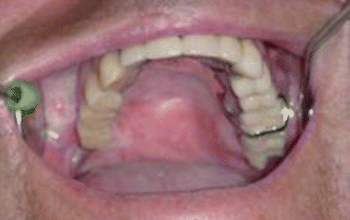


Fig. 2.13
Harvesting myofascial temporalis flap and temporoparietal flap

Fig. 2.14
Early postoperative intraoral view

Fig. 2.15
Mucosalization of the myofascial flap after 6 months

Fig. 2.16
Postoperative view
2.3.3.4 Cervicopectoral Flap
For maxillary defects that require large reconstructions of facial skin, Sakellariou and Salama [42] consider the cervicoparietal flap an excellent alternative that provides an adequate cosmetic appearance and prevents the patchy effect of other techniques. In addition, morbidity of the donor site is minimal, as reported by Shestak et al. [43]. According to Soler-Presas et al. [44], dissection is quick, easy, and safe.
The cervicopectoral flap can be used in combination with other flaps such as the rectus abdominis and temporal myofascial flaps, which provide bulk (Figs. 2.17, 2.18, 2.19, 2.20, 2.21, 2.22, and 2.23).
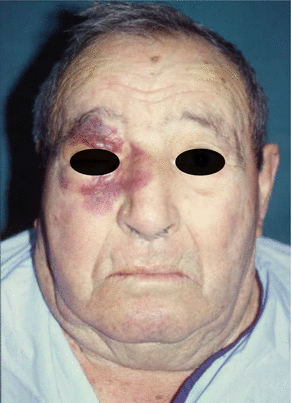
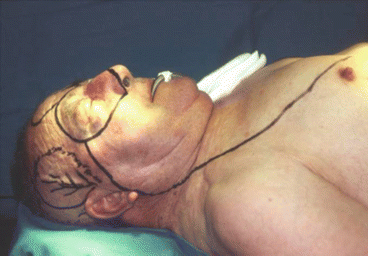
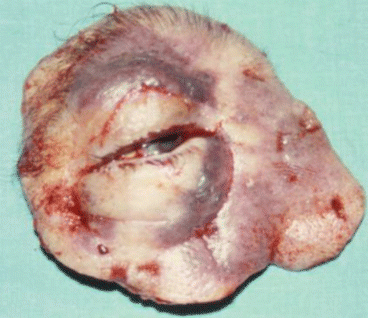
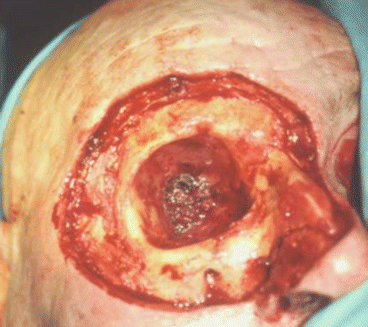
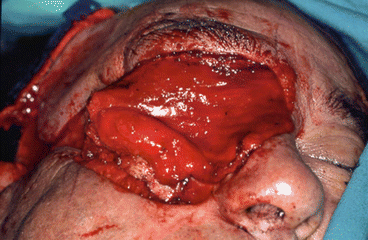
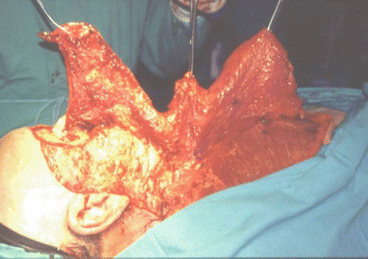
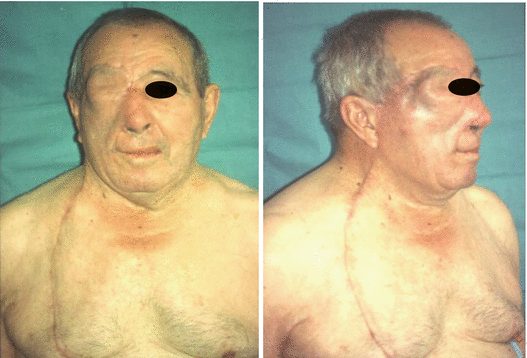

Fig. 2.17
Preoperative view

Fig. 2.18
Intraoperative resection and reconstruction design

Fig. 2.19
Tumor resection

Fig. 2.20
Intraoperative defect

Fig. 2.21
Filling of the orbital cavity with myofascial temporalis flap

Fig. 2.22
Cervicopectoral flap dissection

Fig. 2.23
Postoperative view
2.3.4 Microvascular Free Flaps
In cases where the defect is extensive and involves the palate, with few teeth remaining, Brida et al. [15] recommend surgical reconstruction aimed at a more favorable outcome of prosthodontic treatment in order to guarantee that the patient can speak and swallow again. Therefore, in our hands, free bone flaps are the approach of choice, since they make it possible to fit osseointegrated MG osseous implants and manufacture a prosthesis for dental-alveolar rehabilitation.
During the last 25 years, microvascular free flaps have been a unique alternative for the reconstructive surgeon. Many options are available, depending on the need for soft and bony tissue.
This alternative prevents the length of the vascular pedicle from being a limitation to reconstruction. In addition, it enables the flap to be directed in such a way that it can adapt more accurately to the defect.
Futran [45] proposed the use of microvascular flaps, although no benefit has been shown with a single flap.
Nevertheless, as reported by Kajikawa et al. [46], the main problems are the resulting scar, the variation in the color of the flap, and the difference in texture with respect to the rest of the facial tissue.
Reconstruction of extensive composite defects involving bone, oral mucosa, skin, and soft tissue sometimes requires a combination of flaps, as reported by Wei et al. [47]. Schliephake [48] recommends reconstructing the alveolar process, buttresses, orbital walls, and zygomatic bone using bony components to achieve a satisfactory outcome in the long term.
Kokemueller et al. [49] reported on computer-assisted primary reconstruction of bone contour with tailored titanium implants combined with soft tissue free flaps to fill defects.
2.3.4.1 Rectus Abdominis Flap
The rectus abdominis flap is an easily designed musculocutaneous flap with a large pedicle (inferior epigastric vessels).
Brown type IVA defects are the ideal indication for this flap, which provides the bulk of the missing soft tissue.
We use the rectus abdominis flap in combination with the temporalis myofascial flap: the rectus abdominis provides bulk, and the temporalis muscle is used to reconstruct the soft and/or hard palate defect. It is an excellent alternative to the iliac crest microsurgical flap.
We also use it to provide bulk in type IIIA defects (Figs. 2.24, 2.25, 2.26, 2.27, 2.28, 2.29, and 2.30).
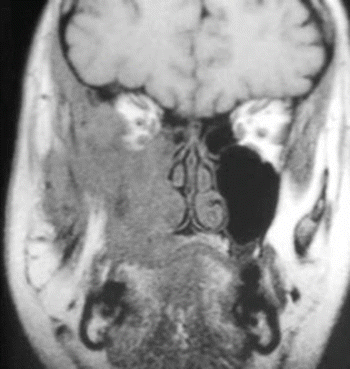
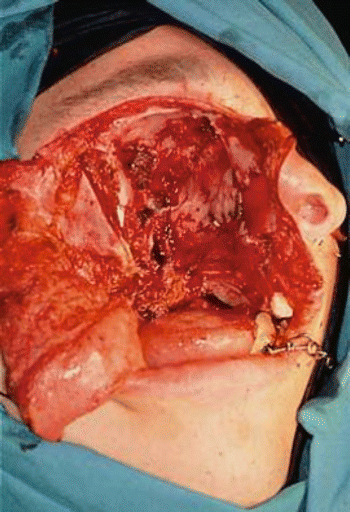
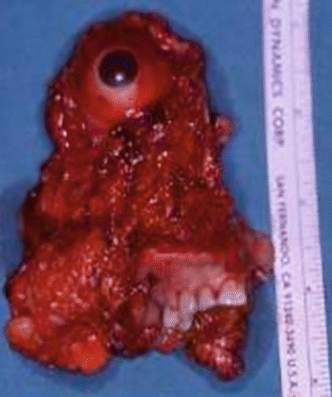
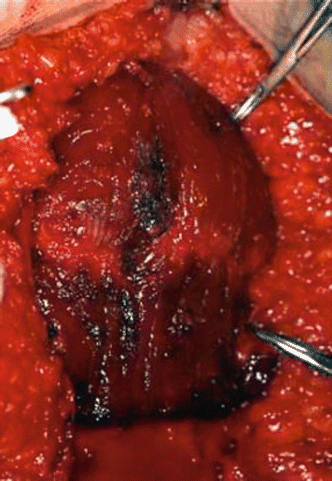
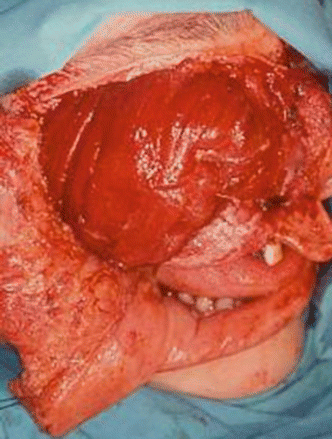
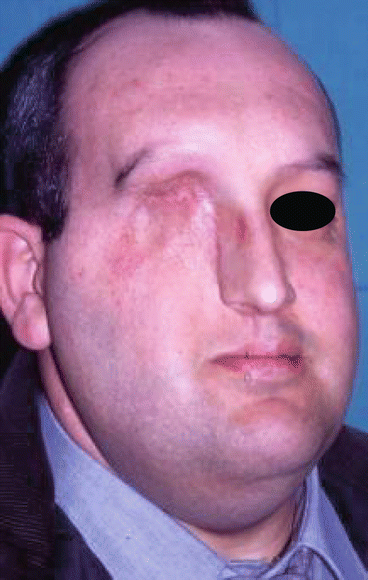
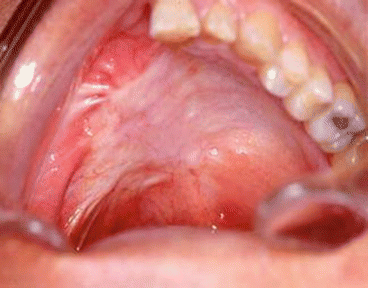

Fig. 2.24
MRI image of the tumor

Fig. 2.25
Surgical defect

Fig. 2.26
Specimen

Fig. 2.27
Harvesting of the rectus abdominis free flap

Fig. 2.28
Position of the flap at the face

Fig. 2.29
Postoperative view

Fig. 2.30
Intraoral aspect of the reconstruction
2.3.4.2 Osteofasciocutaneous Fibula Flap
The first microvascular flap with bone was used by Taylor et al. [50] to treat a post-traumatic tibial defect in 1975. In 1979, Gilbert [51] reported a lateral approach for dissecting the flap. In 1983, Chen and Yan [52] became the first authors to incorporate a skin paddle. However, it was not until 6 years later that Hidalgo [53] used the flap for reconstruction of the mandible.
The constant caliber of the pedicle enabled Wei et al. [54] to the distal end of the fibular vessels as an anastomosis site for a second free flap.
Periosteal supply from the peroneal artery and endosteal blood supply from the nutrient artery, a branch of the peroneal artery, as reported by Pototschnig et al. [55], make it possible to perform multiple osteotomies providing that no damage has been done to the periosteum.
This technique is useful for type Ic and Id defects and is an alternative in Brown type II defects [17]. Reconstruction of soft tissue defects can be achieved using the skin paddle reported by Navarro Vila et al. [56].
Advantages
-
The large quantity of bone available (4 cm up to 25 cm) makes the osteofasciocutaneous fibula flap the largest bone flap, as described by Navarro Cuellar et al. [57].
-
The associated skin paddle can be molded.
-
Two surgical teams can work simultaneously.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses