Fig. 9.1
Schematic representation of the area of positioning of a probe of a laser Doppler flowmetry monitor. The probe is fixed in contact with the buccal surface of the tooth in a rubber split. A schematic representation is given of the technique with a laser beam penetrating through the enamel and the dentine into the pulp, registering the laser Doppler shift
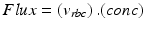
with
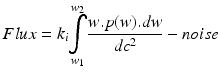
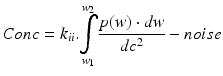
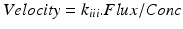
-
with w 1 = lower bandwidth limit (20 Hz)
-
dc = light intensity
-
w 2 = upper bandwidth limit (3,15,22 kHz)
-
dc2 = normalisation
-
w = frequency = weighting factor
-
k i,k ii = scaling constants used for calibration
-
P = power of frequency w
-
noise = dark and shot noise components
Furthermore, pulsations in the blood circulation of teeth are registered in vital elements through the heart-driven blood. The wider the frequency bandwidth, the better another sign of tooth pulp vitality is detected, i.e. the pulse due to the cardiac cycle.
Different wavelengths are used for LDF registrations. Kolkman [45] calculated that the LDF measurement depth is related to the wavelength of the used light. The penetration depth is 2.81 mm in skin tissue with green light (543 nm), 3.14 mm with red light (632.8 nm) and 4.3 mm with almost infrared light (780 nm). These differences in measurement depth as a result of the wavelength used were also confirmed by others, e.g. Fredriksson et al. [46]. When using a 0.5 mm laser beam diameter, the measurement depth for muscle was 0.29 mm (543 nm), 0.62 mm (633 nm) and 0.73 mm (780 nm) and for liver 0.14 mm (543 nm), 0.40 mm (633 nm) and 0.52 mm (780 nm). It is clear that the tissue type is also of importance. Examples of measurement depths shown in the same study [46] were as follows (values are given for a probe-based system with 0.25 mm source-detector separation and an imaging system with a 0.5 mm beam diameter, respectively, both operating at 780 nm): muscle, 0.55/0.79 mm; liver, 0.40/0.53 mm; grey matter, 0.48/0.68 mm; white matter, 0.20/0.20 mm; index finger pulp, 0.41/0.53 mm; forearm skin, 0.53/0.56 mm; and heat-provoked forearm skin, 0.66/0.67 mm. Vongsavan and Matthews [21] found the LDF technique to be capable of making measurements in skin and mucosa up to an approximate depth of 1–2 mm and passing over the 2–3.5 mm-thick enamel and dentine of teeth to measure blood flow to dental tissues, which are more transparent and tubular than dentine and act as light guides. They speculated that the periodontium and other adjacent tissues could contribute to the signal. The penetration depth of LDF beam in teeth for contact and noncontact probe tip was determined by Polat S. et al. [38]. The root was illuminated with a probe in contact to the tooth to 4.28 ± 0.14 mm depth with high density and 13.27 ± 0.27 mm with low density. A noncontact probe illuminated 4.36 ± 0.16 mm of the root with high density and 13.28 ± 0.30 mm of the root with low density. The latter suggests contamination from periodontal tissue blood flow even with adequate precautions.
Gush and King [47] assessed skin blood flow at green and near-infrared wavelengths by spectral analysis; it was concluded that green light was more significantly influenced by capillary blood flow than by larger elements of the microvasculature, due to its much greater absorption in blood, and that the lower frequency components were derived more from capillaries.
9.3 Arbitrary Units
There was also criticism on the laser Doppler technique. Vongsaven and Matthews [21] complained about the nonlinearity of the LDF signal when blood volume fractions in tissue are greater than 1 %, a consequence of the analysis by Bonner and Nossal [48], i.e. for a LDF signal that increases with 100 %, the assumption cannot be made that the blood flow has increased with 100 %. The nonlinearity is explained by the significant number of multiple scattering events of a photon from different red blood cells prior to detection.
A second reason for criticism was the absence of an absolute medium of calibration. The current clinical active calibration is performed with an aqueous suspension of polystyrene microspheres with the probe detecting a reproducible Brownian motion; the calibration fluid is called a ‘motility standard’, and it enables comparisons between measurement over time and between individuals. At present, a representative biological zero for dental pulp blood flow registration is not on the market. A biological zero, in fact, is also not zero, but an every time calibration background reading. Therefore, the latter calibration reference has to take into account the blood flow, the size of the red blood cells and the optical properties of enamel and dentine. This is also the reason for the use of nonstandard ‘perfusion units’ (PU) for LDF and not absolute quantitative measurements in mL/min/100 g.
A third criticism consists of environmental factors. Given that a part of the LDF signal for a dental measurement is of non-pulpal origin, multiple factors will have a significant impact on the LDF readings: the optical properties of the surrounding dentine and enamel (e.g. tooth discoloration), blood circulation of the gingival tissue and every movement of the patient or the probe; patient stress level and the use of medication will also affect pulpal blood flow.
To avoid movement effects, a silicone (dentist) or resin splint (lab) should be made before LDF measurements. The latter is needed for fixation of the LDF probe and should be cleaned with a mild alcohol disinfectant (isopropanol 70 %) in cases of multiple measurements; the splint will also block the ambient light and should help to reduce any influence of gingival blood flow (a green-coloured splint is ideal to block external light). Furthermore, the test area on the tooth (2 mm from the enamel-cement border) [28], the distance (500 μm) between efferent and afferent optic fibres [36], the diameter (200 μm) of each fibre in the probe and the measuring depth in the tissue will affect the obtained values.
9.4 LDF: The Diagnostic Unit
Laser Doppler flowmetry (LDF) provides a non-invasive, objective means of recording blood flow within the teeth, periodontal or bone tissue. LDF measurements are performed using one or more probes, simultaneously [32, 35].
An LDF device with a He-Ne (red, 632.8 nm) or a diode (NIR, 780 nm) laser can be used. The diode laser scores slightly better in specificity and sensitivity [21, 32], probably due to deeper penetration through discoloured teeth. The power of the device is below 2.5 mW with power at the end of the probe typically about1.0 mW (probe diameter = 1.5 mm). For each patient, an opaque splint is used to hold the probe (Fig. 9.1), with measurements made after the acclimatisation period (>10 min). For measurements of tooth vitality, a 3 kHz bandwidth (broad-spectrum, low blood volume) and, for measurements at the gums, a 14.3 kHz bandwidth are proposed. Values are displayed at 40 Hz but can be recorded every 0.1 s over a minimum interval of 2 times 30 s [37].
The location of the probe is determined by the position of the hole made for the probe in the splint, inspected from the inside. The splint is placed in the mouth together with the probe. Each measurement takes at least 30 s, in order to diagnose vasomotor changes, and is repeated two times. A control tooth will always be monitored, if we can measure simultaneously.
To keep additional pulpal components in the readings as low as possible, a rigorous protocol has to be followed. The patient will be seated in a semi-supine position on a dental chair with, for each assessment, the same ambient light. The best diagnostic results will be obtained from healthy, drug-free and relaxed patients. The use of a silicone resin or light-blocking holder, possibly in combination with a rubber dam and at least 2 × 30 s measurement time for each measurement, is highly recommended but time-consuming and therefore not evident. Moreover, there is evidence for diurnal variations. Special attention should be given to testing at the same time of the day when multiple measurements are compared [49].
Finally, the registered data can be processed in a spreadsheet (Excel, Microsoft Corp.) and statistically analysed (SPSS/PC + statistical package, SPSS Inc.).
9.5 Properties
The outgoing flux signal (perfusion units: PU) for a necrotic pulp is on average 42.7 % lower than for a vital pulp [24]. The reason why this is not 100 % relates to the impact of blood circulation in tissues surrounding the measured tooth. This external blood component should be kept as low as possible during registration. Therefore, the position of the probe on the tooth’s surface is of utmost importance.
Measuring simultaneously two contralateral teeth with both LDF probes and equal time variables is highly reliable and recommended [35]. With a ratio of 6:1, the gingival tissue (V rbc = 0.9 m/s) was much better perfused than pulpal tissue (V rbc = 0.16 m/s) [44, 49]. The latter also has an impact on the bandwidth that will be used to measure. Where normal pulp values are measured with a bandwidth of 3 kHz, it is appropriate for gingival tissue to measure at 14.3 kHz. Gingival tissue has an increased flow of red blood cells.
Yet one must also make the objection that 80 % of the LDF signal of an intact tooth, measured without rubber dam, does not originate from the pulp. This non-pulpal component drops to 43 % with rubber dam. Soo-ampon et al. therefore suggest that 43 % corresponds to the mathematical noise and environmental impact [37]. According to Wannfors and Gazelius, even 5 % of this non-pulpal component originates from circulation of the bone [50]. Studies conducted by the group around Polat already give a reason for the extra-large pulpal influence [38]. They found reflections well beyond the dental tissues and attributed this to the high intensity of the laser light and its penetration through dental tissues [38, 40].
9.6 LDF: A Benefit?
Dental pulp testing is an essential aid in endodontics. At present, most dentists rely on thermal tests and to a lesser extent on electric pulp testing to evaluate the ‘pulp vitality’. These tests, however, are sensibility tests and do not provide information about pulp vitality (blood supply) and/or not decisive enough to determine whether a tooth is necrotic [51, 52]. False responses may occur for both. Especially, thermal tests are used to diagnose the diseased tooth and to reproduce the disease state [51]. The result is that misdiagnosis may lead to incorrect, inappropriate or unneeded treatment [53].
LDF has been shown to be more reliable than thermal testing, electric pulp testing and the use of pulse oximetry [54–56]. Compared with normal ‘sensitivity testing’, LDF is more reliable. When LDF scores 1.0 for sensitivity and 1.0 for specificity regarding vital or non-vital pulp tissue, a cold test with ethyl chloride scores 0.92 and 0.89 and an electric pulp test 0.87 and 0.96 [33]. The evaluation of heat versus cold test and electric test as a measure for pulp vitality gave a negative result in the case of pulp necrosis for the cold test in 89 %, with the heat test in 48 % and with the electric pulp test in 88 % [53]. In the same investigation, a positive cold response was given in 90 % of all cases for a ‘vital pulp’, 83 % with heat and 84 % with the electric pulp test [57]. For LDF, in both cases, this was 100 %. It will take traumatised pulps at least 6 weeks for a sufficient recovery of sensation [58, 59]. Therefore, standard sensitivity tests will provide ‘reliable’ information at the very best 6 weeks after traumatic impact, whereas one can determine the state of tooth vitality immediately after trauma with LDF.
Moreover, it was confirmed with LDF in an orthodontic surgery study that a tooth without normal innervations may have an intact blood supply despite negative response to the classic tests [45]. LDF registrations have been shown to follow the pattern of wound healing in both a non-induced trauma (Luxation) and a well-induced trauma (Le Fort I osteotomy) [35, 44].
As previously shown, there was already abundant proof in the 1980s and the 1990s that LDF could be used to evaluate and follow-up the restoration of ‘vitality’ in traumatised elements [17, 18, 20, 24, 25, 33].
LDF is therefore a technique that allows the practitioner to establish an early reliable diagnosis enabling correct, appropriate and not too early treatment taking into account the limitations of thermal and electric tests [41–43]. A two-probe assessment is advisable for giving instant information when comparing two teeth with LDF (Fig. 9.2) [32, 41].
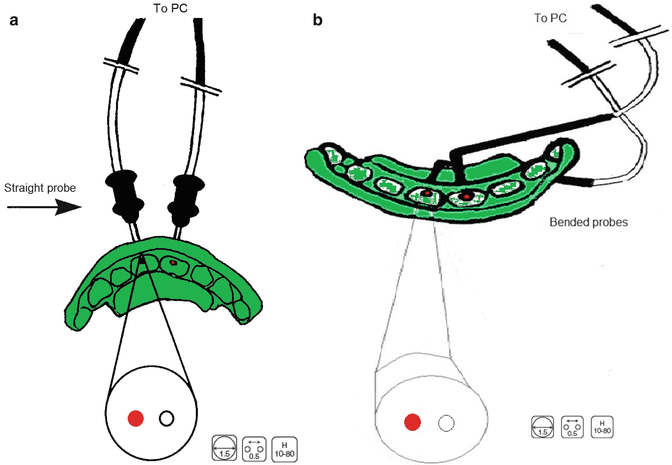

Fig. 9.2
Schematic presentation of a two-probe laser Doppler flowmetry assessment with straight and angulated probes. The latter are used when it is impossible to position straight probes. With this set-up, it is possible to register the blow flow in two teeth simultaneously, e.g. the traumatised tooth and its antimere. (a) straight probes positioned at the buccal side; (b) bended probes positioned at the palatal side
9.7 Restoration of Tooth Vitality (Blood Flow) and Revascularisation
Multiple articles comment on recovery, rehabilitation or restoration of the blood circulation/blood flow and also on revascularisation after traumatic injury in different areas of medicine. All authors report on three major periods in the recovery of the blood flow: (1) the period immediately after trauma and an initial, brief period of hyperaemia; (2) a consecutive period of ischaemia; and (3) the restoration of the vascularisation. These periods vary according to the function of the respective tissue and the extent or severity of the tissue damage.
The evaluation of wound healing and revascularisation especially of burns turns out to be a major field of use for LDF. Here, the recovery pattern is extended to even 1 year. In this respect, LDF is the perfect example of a non-invasive evaluation technique without any need of injection of contrast agents to visualise the vessels.
Likewise, as in clinical medicine, evaluation of revascularisation is needed in a number of dental situations. In the situation of dental trauma and pulp injury, LDF is essential for the assessment of blood perfusion and will be used besides sensibility tests. Slightly different outcomes have been registered for non-induced and induced traumata with varying recovery periods [7, 41]
Three trauma cases where LDF was used to monitor the pulp vitality are now described.
9.7.1 A Complicated Trauma Case
One of the first cases that we monitored with LDF was published in 1999 [35]. It reported on a 24-year-old Caucasian female being hit by a moped driver on a footpath. The victim was treated at the emergency department of the Ghent University Hospital (Belgium) after the accident. Both central incisors (I) and the right canine (C) of the upper jaw were luxated and involved alveolar bone fracture situated above both central incisors and the left lateral incisor (detected clinically and radiographically). Moreover, the left central incisor was intruded over ±7 mm. After the repositioning of the alveolar bone segment and the maxillary left central incisor, a rigid splint was fixed for 3 weeks (a therapy that is no longer performed nowadays). The patient was then referred to the department of endodontology for further systematic clinical follow-up.
All maxillary front teeth were screened on six successive occasions over 30 weeks with current sensitivity tests, i.e. CO2 ice (Miracold Plus®, Hager & Werken, Duisburg, Germany), heat (gutta-percha), electric pulp test on the buccal surface of each tooth (Dentotest®, Malek, Switzerland) and LDF as test of vascularisation. Four years later, a similar assessment was repeated. The patient was in good health on each occasion and did not take any form of medication. The teeth were examined by the same investigator under standardised environmental conditions. On each occasion, two registrations were taken with an interval of 5 min. The colour of all teeth remained normal and the incisors remained untreated. Radiographs were made in order to distinguish intra-pulp and peri-radicular pathology as a part of the systematic follow-up. No periodontal disease was found.
According to the method described by Roeykens et al. [35], LDF evaluation was performed using a DRT 4 LDF monitor (Moor Instruments Ltd., Axminster, Devon, England) with a laser diode at 780 nm, and a probe output of 1.0 mW was used. The manufacturer ensured an output between 0.1 and 0.5 PU from a static reflector. The DRT 4 recorded tooth signals at a bandwidth of 3 kHz and with a time constant of 0.1 s. The display rate was set on 40 Hz with a time span of 65 s. Each probe (∅ = 1.5 mm, two optical fibres with diameter = 0.2 mm at 0.5 mm separation of centres) was labelled and calibrated following the manufacturer’s instructions. An LDF signal was simultaneously obtained from both comparable teeth using two identical probes allowing for instant comparison.
The probes were fixed in a green rubber base reposition splint (Exaflex® GC) to the buccal enamel surface 2 mm from the gingival margin. The reposition splint was removed from the mouth between all measurements, disinfected with an alcohol solution and slightly adapted with Exaflex® on the second occasion after debonding of the rigid splint. This caused no significant alterations in the recorded signal.
The values obtained with the sensibility tests (CO2 ice and gutta-percha) were negative for all teeth involved in the trauma until the ninth week (Tables 9.1 and 9.2); electric pulp testing showed significant values from the seventh week (Table 9.3). All parameters, except the electric pulp test for tooth 11 (value 6 on a scale of 10 after 4 years), returned to a normal status at the end of this follow-up. The hand temperature remained constant 35.9 °C (±0.1) (Tables 9.4 and 9.5).
Table 9.1
Registration of the sensibility of the maxillary front teeth after exposure to heat
|
Tooth/week
|
Heat
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
1 week
|
2 weeks
|
3 weeks
|
7 weeks
|
9 weeks
|
20 weeks
|
30 weeks
|
228 weeks
|
|
|
Left canine
|
(−)
|
Late
|
Late
|
±
|
±
|
nl
|
nl
|
nl
|
|
Left lateral incisor
|
(−)
|
(−)
|
(−)
|
(−)
|
(−)
|
nl
|
nl
|
nl
|
|
Left central incisor
|
(−)
|
(−)
|
(−)
|
±
|
±
|
Late
|
nl
|
nl
|
|
Right central incisor
|
(−)
|
(−)
|
(−)
|
±
|
±
|
Late
|
Late
|
nl
|
|
Right lateral incisor
|
nl
|
nl
|
nl
|
nl
|
nl
|
nl
|
nl
|
nl
|
|
Right canine
|
(−)
|
(−)
|
(−)
|
(−)
|
(−)
|
Late
|
Late
|
nl
|
|
Morning
|
Noon
|
Afternoon
|
Night
|
|
|---|---|---|---|---|
|
Control
|
18.88
|
14.25
|
14.18
|
9.57
|
|
±
|
1.47
|
1.23
|
1.2
|
1.42
|
|
30 dentists
|
18.79
|
15.09
|
14.98
|
|
|
±
|
1.54
|
1.3
|
1.4
|
Table 9.2
Registration of the sensibility of the maxillary front teeth after exposure to cold
|
Tooth/week
|
Cold
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
1 week
|
2 weeks
|
3 weeks
|
7 weeks
|
9 weeks
|
20 weeks
|
30 weeks
|
228 weeks
|
|
|
Left canine
|
(−)
|
Late
|
(−)
|
±
|
±
|
nl
|
nl
|
nl
|
|
Left lateral incisor
|
(−)
|
(−)
|
(−)
|
(−)
|
(−)
|
Late
|
nl
|
nl
|
|
Left central incisor
|
(−)
|
(−)
|
(−)
|
±
|
±
|
Late
|
nl
|
nl
|
|
Right central incisor
|
(−)
|
(−)
|
(−)
|
±
|
±
|
Late
|
Late
|
nl
|
|
Right lateral incisor
|
nl
|
nl
|
nl
|
nl
|
nl
|
nl
|
nl
|
nl
|
|
Right canine
|
(−)
|
(−)
|
(−)
|
(−)
|
(−)
|
Late
|
Late
|
nl
|
Table 9.3
Registration of the effect of electric pulp testing on the sensibility of the maxillary front teeth
|
Tooth/week
|
Electric pulp test (mean value 3 measurements, SD) scale 1–10
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
1 week
|
2 weeks
|
3 weeks
|
7 weeks
Stay updated, free dental videos. Join our Telegram channel
VIDEdental - Online dental courses
 Get VIDEdental app for watching clinical videos
Get VIDEdental app for watching clinical videos

| |||||