Fig. 13.1
(a) Custom made device fabricated for tethering the strained fibrin gel. (b) The representative SEM image of microstructure of fibrin gel without strain (Bar: 1 μm). (c) The representative SEM image of strained fibrin gel (Bar: 5 μm). (d) The direction of cell proliferation was also identical with strain direction of hydrogel (Bar: 100 μm). (e) Three-dimensionally aligned cell groups formed in the strained fibrin gel (Green; actin, Blue; nucleus) (Bar: 400 μm). (f) Three- dimensionally aligned cell groups formed in the strained fibrin gel (Hematoxylin–Eosin staining) (Bar: 200 μm). (g) SEM images of aligned cells in the strained fibrin gel (Bar: 100 μm)
13.3 Three-Dimensional Patterning of Mineralized Cell Groups in Hydrogel
The cell dynamics within the strained fibrin gel were investigated. The fibrinogen solution containing myoblast (C2C12) was used to form a gel, which was continuously subjected to 25 % strain. The cells in the fibrin gel display a specific alignment, that is, parallel to the strain direction. Interestingly, the direction of cell proliferation was identical to that of cell alignment (Fig. 13.1d). A single seeded cell therefore divided multiple times, and the oriented cells subsequently formed linear groups aligned parallel to the strain direction (Fig. 13.1e, f), in a similar manner to the cellular organization found in a longitudinal section of native skeletal muscle tissue. It is assumed that the positions of the cells in the fibrin gel are restricted to the spaces between the fibrin bundles such that they align and proliferate parallel to the strain direction. This assumption is supported by a typical SEM image (Fig. 13.1g), which shows cells positioned in the spaces between the bundle-like structures of the fibrin gel.
In light of the above-described results, bone-marrow stromal cells (BMSCs) we used instead of myoblasts to fabricate the aligned mineralized tissue. In a similar manner to the cells in the above-described myoblast study, the cells in the strained gel displayed a specific alignment, namely, parallel to the strain direction in the gel. The direction of cell proliferation was identical to that of cell alignment. Consequently, the oriented cells formed a number of linear cell groups aligned parallel to the strain direction in the strained gel. In the strained fibrin gel, type-I collagen deposition showed a specific orientation, namely, parallel to the strain direction (Fig. 13.2a, b). The merged images with nuclear-stained cells in the figure indicate that the matrix was deposited in identical positions to the cell positions in the gel. When BMSCs were cultured in an osteogenic differentiation medium, mineralization derived from the cells was observed in the fibrin gel. Similar to the matrix deposition, mineral deposition was localized according to the cell position and showed a specific alignment in the strained gel. X-ray-diffraction (XRD) peak profiles revealed that the obtained mineral in the fibrin gel was hydroxyapatite (HAp) (Fig. 13.2c). Additionally, a specific orientation of the HAp crystals (namely, parallel to the strain direction) was confirmed from the relative intensity of the (002) to (211) planes in the XRD profiles (Fig. 13.2c). SEM images indicate that compared to those in the control gel, the mineralized matrix vesicles were concatenated serially parallel to the strain direction in the strained gel (Fig. 13.2d). EDS analysis revealed that the mineralized matrix vesicles contain both calcium and phosphorus. The concentrations of phosphorus in the mineralized vesicle regions are much higher than those of calcium in that region.
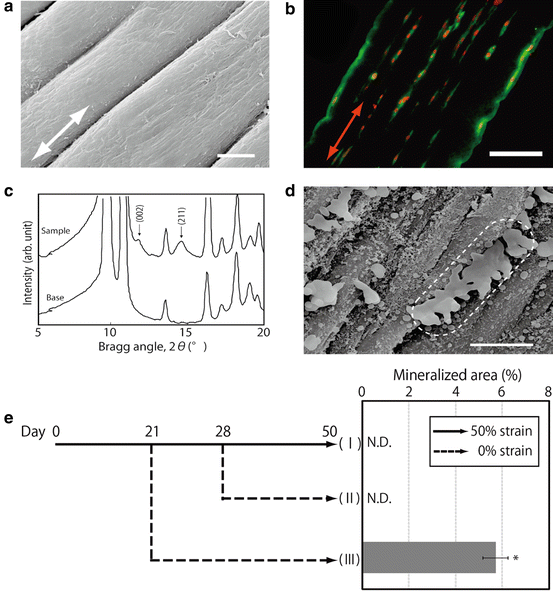

Fig. 13.2
(a) SEM images of bundle-like structure formed in the strained fibrin gel (Bar: 20 μm). (b) Aligned cell groups and precipitated type I collagen according to the cell group presence (Bar: 250 μm). (c) X-ray diffraction analysis of precipitated minerals within the strained fibrin gel. (d) Connected minerals on aligned cells and fibers (Bar: 10 μm). (e) Mineralized area in strained fibrin gel cultured with different strain conditions. (I) 50 % strain for 50 days, (II) 50 % strain for 28 days reduced to 0 % strain for another 22 days, and (III) 50 % strain for 21 days reduced to 0 % strain for another 29 days (*: p < 0.05)
To investigate the alternations of cellular functions in strained fibrin gel, the cells were cultured in gels with different strain rates. At day eight, the gel subjected to a higher strain rate had enhanced cell proliferation compared to the gel subjected to a lower strain rate. The mRNA expressions of Opn and Oc, namely, osteogenic differentiation markers, were investigated at day four. Both Opn and Oc expressions decreased with the increasing strain rate from up to 50 %. These results suggest that cell functions in the strained fibrin gel are regulated by the alteration of strain rate. To confirm this suggestion, cell-derived mineralization in the gel (subjected to varied strain rate during the culture period) was investigated. Mineralization caused by cell differentiation was detected only in the sample that was subjected to strain rate decreased from 50 to 0 % at day 21 (III). In contrast, mineral deposition was not detected in the gel that was subjected to strain rate reduced from 50 to 0 % at day 28 (II) or in the gel that was subjected to 50 % strain maintained for 50 days (I) (Fig. 13.2e) [14].
13.4 Microvessel Patterning Using Fibrin Gel with Dynamic Mechanical Stimulation
By here, the cells were cultured in the strained condition; however, the strain was static and continuous. A device for applying dynamic strain against to cells in 3D hydrogel was also designed. The device was used to investigate the formation of vascular tissue under cyclic mechanical stimulations. Dextran beads coated with human umbilical endothelial cells (HUVECs) were embedded in fibrin gels within a custom 3D chamber and subjected to cyclic strain (Fig. 13.3a, b). HUVECs on the beads started to invade the gels in a direction perpendicular to the strain direction and formed sprout-like structures within 5 days (Fig. 13.3c
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


