Important amino acid
% of total amino acids
Function
Glycine
33
Enhances van der Waals forces and hydrogen bonds that hold three helical polypeptides together.
Proline
14
Responsible for the extended helix.
Hydroxyproline
9
Hydroxyl group stabilizes the extended helix at higher temperatures.
Alanine
12
Small side chain allows polypeptides to lie alongside each other and form fibers.
Serine
4
Small side chain (same effect as alanine).
Lysine
3
Responsible for covalent cross-linking.
Hydroxylysine
1
Attaches carbohydrate and is involved with lysine in covalent cross-linking.
Collagen fibrils, fibers and fiber bundles may be isolated from calf skin dermis by disaggregation with a neutral salt buffer followed by differential centrifugation. The pure fibers are boiled in sodium dodecyl sulfate (SDS) and subjected to polyacrylamide gel electrophoresis (SDS-PAGE). In this modern system, the collagen polypeptides are separated by molecular weight. Figure 4.1 shows that collagen fibers are composed of α1-, α2-, β-, and γ- polypeptides called tropocollagen. Marker proteins of known molecular weight indicate that the smallest polypeptides α1– and α2-tropocollagens are both approximately 100 kDa; the α1-polypeptide being slightly larger because it migrates less. The origins of the β- andγ- chains are discussed below (Sect. 4.2.2).


Fig. 4.1
The polypeptide composition of collagen fibers. Periodontal membrane fibers were dissected, boiled with sodium dodecyl sulfate (SDS), in the presence of mercaptoethanol to provide a reducing environment, and separated by polyacrylamide gel electrophoresis (PAGE). Polypeptides were visualized by staining with a dye after electrophoresis. The smallest polypeptides migrate most from the origin. The α-tropocollagen chains are about 100 kDa mol wt, the α2-chain being slightly smaller (nearer the foot of the gel) than the α1-chain. The β-bands have a molecular weight of about 200 kDa. The β1, 2-band consists of an α1-chain covalently cross-linked to an α2-chain; the β1, 1-band consists of two cross-linked α1-chains. The γ-tropocollagen chain has a molecular weight of 300,000 and consists of three covalently cross-linked α chains (Adapted from Fig. 2.7 published in: Biochemistry, A Case-Oriented Approach, 4th Edition. R. Montgomery, R.L. Dryer, R.C. Conway, and A. Spector, C.V. Mosby Co., St Louis, MO 1983; Copyright Elsevier, 2008)
The α1 and α2-polypeptides may be cut out of the gel, each partially digested with a suitable protease and the fragments separated, blotted onto a membrane and the first 18–23 N-terminal residues of each peptide sequenced. After sorting for overlaps, large segments of the sequence may be obtained. The results consistently indicate that glycine is present at every third residue (Gly-X-Y repeating motif) in both α-polypeptides and that proline is often present at the X or Y position. Other studies indicate that artificial polypeptides containing a Gly-X-Pro repeating sequence have an extended (unfolded) chain due to the conformation of the proline peptide bond (Fig. 4.2), a left-handed extended helix called the collagen helix (Fig. 4.3) because of its presence in all types of collagen.
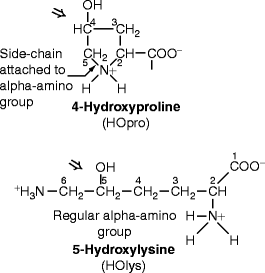
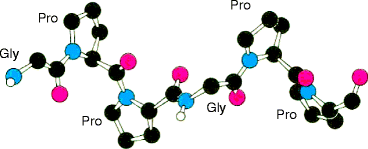

Fig. 4.2
Structures of hydroxyproline and hydroxylysine

Fig. 4.3
Collagen helix. This secondary structure is created by peptide bond conformation around the proline and hydroxyproline residues (From Fig. 2-39 in Biochemistry. L. Stryer, 4th Ed. 1995. W.H. Freeman & Co., New York)
This left-handed secondary structure of collagen polypeptides differs from the α-helix, whose tightly coiled, right-handed helix is disrupted by proline. It also differs from a β-sheet, a series of six to ten amino acids in extended chain configuration in which proline residues create sharp turns, allowing the chains to lie alongside each other. The secondary structure of a fibrous collagen α-polypeptide is an extended helical rod (Fig. 4.4a and b). The glycine residues permit strong associations between the peptides (quaternary structure) due to hydrogen bonding between the hydrogen atom residue of glycine and carbonyl and amide groups of nearby peptide bonds. Tertiary structure is absent. The result is an extended triple helix, tropocollagen (Fig. 4.4c), which forms the monomeric unit of all fibrous collagens. Figure 4.5 is a cut-through view of the chains in Fig. 4.4. It shows a central cavity, across which the glycine hydrogen atom (glycine side-chain) holds the three α-polypeptides together by hydrogen bonding between alternate pairs of the chains.
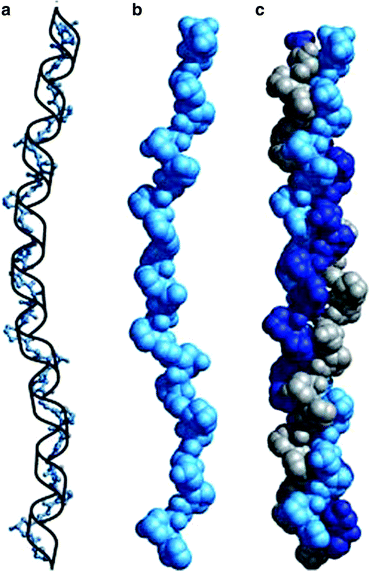
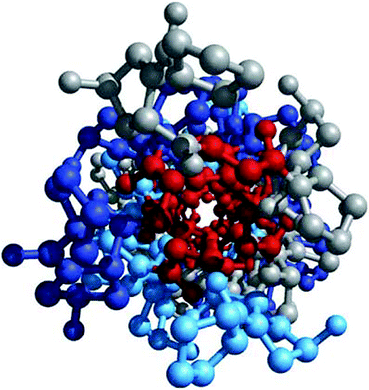

Fig. 4.4
Collagen triple helix formation. (a) A repeating tripeptide sequence Gly-X-Pro or Gly-X-4-Hyp adopts a left-handed helical structure with three residues per turn. The repeating sequence used to generate this model is Gly-Pro-4-Hyp. (b) Space-filling model of the same chain. (c) Three of these chains (gray, blue, and purple) wrap around one another with a right-handed twist (Figure 4.12a, b and c in Lehninger Principles of Biochemistry. D.L. Nelson and M.M. Cox, 4th Ed. 2005. W.H. Freeman & Co., New York)

Fig. 4.5
Glycine residues make up the interior of a tropocollagen triple helix. The same three-stranded collagen super-helix is depicted as in Fig. 4.4, but looking down the center of a ball-and-stick representation. Glycine residues (−H) are shown in red. Because of its small size, glycine is required where the three chains contact. The balls in this illustration do not represent the van der Waals radii of the individual atoms (Figure 4-12d in Lehninger Principles of Biochemistry. D.L. Nelson and M.M. Cox, 4th Ed. 2005. W.H. Freeman & Co., New York)
Recently, fluoroproline, made by artificially replacing the hydroxyproline hydroxyl group with a fluoride atom, was found to form a collagen helix that was more stable to heat denaturation than hydroxyproline or proline. These studies also indicated that fluoroproline and hydroxyproline stabilize the collagen helix to heating by promoting an extended (trans) form of the peptide bond. Other studies indicate that a proline helix becomes unstable above 10°C, but that replacing about half of the proline residues with hydroxyproline as in mammals (Table 4.1) stabilizes the helix at 37°C. In cold-blooded vertebrates such as fish, the tropocollagen must dissociate at 20°C or lower, not at 37°C. Less hydroxyproline is present in cold-water fish collagens. These studies suggest that it is the secondary structure (the collagen helix) that stabilizes the triple helix to heat denaturation. Quaternary structure hydrogen bonds involving the OH group of hydroxyproline or the side chain of glycine to carbonyl groups of the peptide bond seem less important.
After its expression from fibroblasts, the α-polypeptides of tropocollagen form fibers spontaneously (without an enzyme). The standard model of tropocollagen is derived from peptides such as (pro-pro-gly)n that suggest a helical repeat length of 30 amino acid residues. Unfortunately, this finding is inconsistent with X-ray diffraction patterns from native collagens, which suggest a repeat length of only 21 amino acids. Indeed, recent analysis of nonpolar residues (Val, Leu, Ile, Met, and Phe) in the α1 tropocollagen sequence of the most common form of collagen fiber reveals them to predominate near the centers of 21-residue segments. The spontaneity of collagen fibril assembly may be initiated by hydrophobic interactions between tropocollagen molecules at these centers within the extended chains and that this guides the subsequent, stabilizing formation of the glycine-bonded triple helix.
The alpha chains of fibrous collagens (tropocollagen) have many proline (and hydroxyproline) residues responsible for the collagen helix, an extended chain, left-handed helix (secondary structure) different from the α-helix and β-sheet in other proteins. Glycine and hydroxyproline are responsible for the association of the polypeptides into a triple helix. The glycine side chains (hydrogen atoms) hydrogen bond to carbonyl groups of a nearby peptide bond in the helical backbone and hydroxyproline OH groups to amide groups of other nearby peptide bonds. The hydroxyproline bonds enhance triple helix formation at 37°C. Cold-blooded vertebrates have less hydroxyproline in their collagens (non-fibrillar as well as fibrillar). Serine and alanine have short side chains that allow the three chains to come together more easily than long residues. Recent X-ray diffraction studies of native collagen suggest that a 21 amino acid repeating unit whose central region contains hydrophobic amino acid residues initiates triple helix formation.
4.2 Collagen Fiber Formation
The α1– and α2– tropocollagen polypeptides are each a long, central portion (domain) of two larger polypeptides (procollagen) encoded by genomic DNA (genes COL1A1 and COL1A2). The sequences of the respective genes are similar (homologous). And assemble so that two α1– and one α2-tropocollagen polypeptides interact by their hydrophobic domains and initiate triple helix formation. The tropocollagen polypeptides are then cleaved out as the fibril-forming monomeric unit (Fig. 4.6). The removed portions are called the collagen propeptides. The N- and C-terminal ends of the excised central domains are called the tropocollagen telopeptide domains. These telopeptide domains interact with adjacent tropocollagen molecules (yellow lines in Fig. 3.3) so that a staggered array forms with gaps (bottom half of Fig. 3.3). The one-quarter overlap of the arrayed molecules causes a striated appearance, the dark and light banding pattern at the top of Fig. 3.3. The gaps also control how the fibers stain with dyes, cross-link, calcify and degrade (Table 4.2). Figure 4.7 shows how the quarter-staggered arrays aggregate into fibrils (thin, small fibers <30 nm diameter) and fibers (>300 nm diameter). Filaments and shorter length microfibrils are thin, extended aggregates (>10 nm diameter).
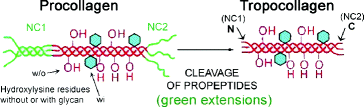

Fig. 4.6
Procollagen is the precursor of tropocollagen. Tertiary structures are absent from tropocollagen, but present on procollagen whose N- and C-termini fold like a regular protein. These globular non-collagenous (NC) domains at each end of the procollagen molecule (green) form soluble, tertiary structures that keep the central, glycine/proline-rich (collagenous) domain in solution until secreted from the cell. These domains are called propeptides. The C-terminal NC domain is usually referred to as NC1 and the N-terminal domain as NC2. In non-fibrillar collagens, and in fibrillar collagens with interrupted collagenous domains (see Table 3.1), there are additional NC domains which are numbered so that the C-terminus propeptides remains NC1. For example, the N-terminal domain of type IX collagen in cartilage is NC4 (see Fig. 6.14a). The propeptides are removed from fibrillar procollagens after secretion to form α-tropocollagen (red, right side of figure), but remain uncleaved in non-fibrillar collagens. The N- and C-terminal ends of α-tropocollagen are also non-helical and referred to as the telopeptides (N and C ends of red portion on right side of figure). Telopeptides are involved in cross-linking the fibers (see Fig. 4.9) (Modified from Fig.19-47 in The Molecular Biology of the Cell, B. Alberts et al., 4th Ed. 2002. Garland Science, Taylor & Francis Group, New York)
Table 4.2
Functions of gaps in the fibrillar collagen array
|
1.
|
Dye staining gives the microfibril a striated appearance under the electron microscope.
|
|
2.
|
Lysine residues predominate around the gaps, causing glycan attachment and polypeptide cross-linking.
|
|
3.
|
Phosphorylation nucleates calcium as bone forms.
|
|
4.
|
Site of initial collagenase degradation.
|
|
5.
|
Site of fiber interactions with non-fibrous collagens
|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


